Introduction
The Sibumasu Block, located at the eastern part of the Cimmerian belt (Şengör, Reference Şengör1984), is a key terrane in interpreting the tectonic and paleogeographic evolution of the Paleotethys, Mesotethys, and Neotethys. It has been widely accepted as a single, north-to-south trending, Permian tectonic entity that included the “Shan States of Burma, northwest Thailand, Peninsular Burma, southern Thailand, western Malaya and Sumatra” and also possibly extended northward into the southwestern part of Yunnan and Tibet (Metcalfe, Reference Metcalfe1984, p. 107). This block was proposed mainly on the grounds of the presence of massive and extensive glacial-marine diamictites in these areas (Metcalfe, Reference Metcalfe1984, p. 116). However, true glacial-marine deposits are rare and relatively thin in some parts of the Sibumasu including the Malaysian part of the block, southern Peninsula, and central Thailand (e.g., Stauffer and Lee, Reference Stauffer and Lee1986; Ridd, Reference Ridd2016), and are little or poorly known from the main Shan Plateau in Myanmar so far (e.g., Thura Oo et al., Reference Oo, Tin and Nyunt2002). Earlier, Bender (Reference Bender1983) worked in Myanmar and noted the different stratigraphic successions between the western Shan Plateau and areas further east and named the former the Karen-Tenasserim Unit. Mitchell et al. (Reference Mitchell, Hlaing, Htay, Mantajit and Potisat2002) later adopted the term Slate Belt for that unit. In Thailand, Ridd (Reference Ridd2009) and Ridd and Watkinson (Reference Ridd and Watkinson2013) noted the same west-to-east contrasting stratigraphic successions, calling the western belt the Phuket Terrane, which these authors thought continued into Myanmar as the Phuket-Slate Belt terrane. More recently, Ridd (Reference Ridd2016) introduced a new terrane into Southeast Asia, named the Irrawaddy Block. According to that author, the Irrawaddy Block encompasses the Phuket-Slate Belt and represents the southern extension of the Lhasa Block in Tibet and the Tengchong Block in the western Yunnan of China, on the basis of their attaining comparable thickness and types of glacial-marine deposits (Fig. 1.1). In such a model, a late Paleozoic-Mesozoic ocean was suggested to exist between the Irrawaddy Block and the remaining parts of the Sibumasu Block (i.e., the Sibuma Block), and this ocean, called Thai-Myanmar Mesotethys, is probably the southern extension of the Bangong-Nujiang Ocean in Tibet (Ridd, Reference Ridd2016). On the contrary, Metcalfe (Reference Metcalfe2017) considered the Sibumasu Block to be a single unit and favored no suture zone within it.
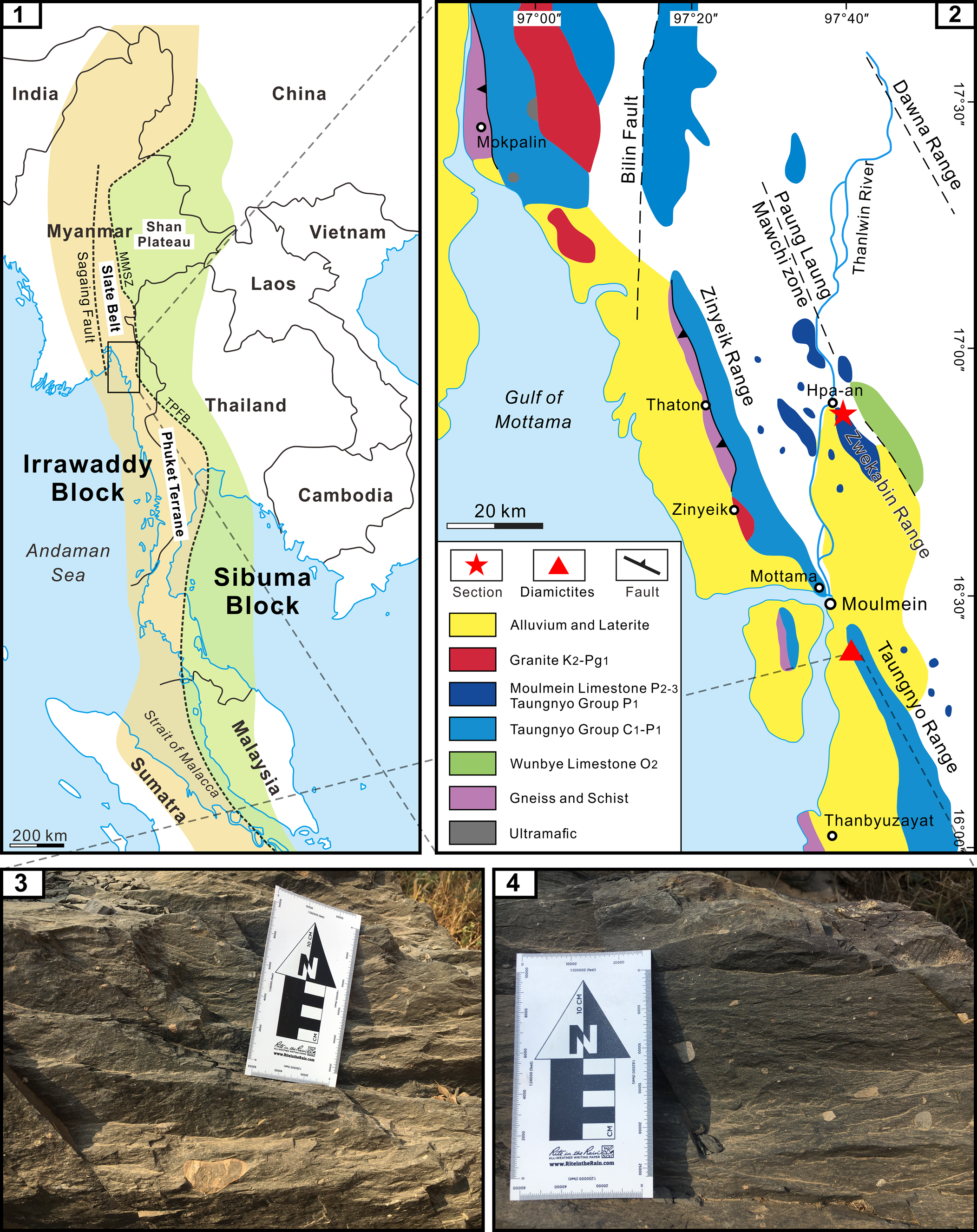
Figure 1. (1) Distribution of the Irrawaddy and Sibuma blocks in Southeast Asia, modified from Ridd (Reference Ridd2016); MMSZ = Medial-Myanmar Suture Zone; TPFB = Three Pagodas Fault belt. (2) Geologic sketch map of the study area with locations of the Kya-in Taung section and diamictites, modified from Mitchell (Reference Mitchell2018). (3, 4) Glacial diamictites of the Taungnyo Group.
The Sibumasu Block has been overwhelmingly regarded as having rifted from the northern peri-Gondwanan margin during the early–middle Permian. The Permian lithofacies and climate-sensitive organisms in the block, e.g., brachiopods and fusulines, offer the most insightful data to clarify its paleobiogeographic, paleogeographic, and tectonic evolution, which will further help solve the problem of possible tectonic subdivisions within the Sibumasu Block. It has been widely recognized that the Cisuralian deposits of the Sibumasu Block are characterized by glacial-marine diamictites containing typical cold-water biotas (Shi and Archbold, Reference Shi and Archbold1995; Metcalfe, Reference Metcalfe2002, Reference Metcalfe2011; Ueno and Charoentitirat, Reference Ueno, Charoentitirat, Ridd, Barber and Crow2011; Ridd, Reference Ridd2016). Above the glacial-marine diamictites, marine faunas and their paleobiogeographic affinities progressively changed in the Permian during the northward drifting of the Cimmerian continents (Shi and Archbold, Reference Shi, Archbold, Hall and Holloway1998; Jin, Reference Jin2002; Ji et al., Reference Ji, Yao, Jin, Yang, Wang, Yang and Wu2004; Shi et al., Reference Shi, Huang, Jin and Yang2011; Shen et al., Reference Shen, Zhang, Shi, Li, Xie, Mu and Fan2013; Zhang et al., Reference Zhang, Shi and Shen2013, Reference Zhang, Shi, Shen and Yuan2014; Ueno et al., Reference Ueno, Arita, Meno, Sardsud and Saesaengseerung2015; Yuan et al., Reference Yuan, Zhang, Shen, Henderson, Zhang, Zhu, An and Feng2016; Xu et al., Reference Xu, Zhang, Qiao and Shen2019), as well as paleoclimatic amelioration from an icehouse to a greenhouse regime (Chen et al., Reference Chen, Joachimski, Shen, Lambert, Lai, Wang, Chen and Yuan2013; Zhang et al., Reference Zhang, Shi and Shen2013; Haig et al., Reference Haig, Mory, McCartain, Backhouse, Håkansson, Ernst, Nicoll, Shi, Bevan, Davydov, Hunter, Keep, Martin, Peyrot, Kossavaya and Santos2017; Liu et al., Reference Liu, Jarochowska, Du, Munnecke and Dai2017). Thus, it can be assumed that the faunas and sedimentary sequences are different if the Irrawaddy Block and Sibuma Block had different tectonic and paleogeographic evolutionary histories. However, fossil records with clear paleobiogeographic implications are very rare in Myanmar; in particular, fossils from the Irrawaddy Block are basically lacking.
Here, we report a late Cisuralian brachiopod fauna from the uppermost part of the Taungnyo Group in eastern Myanmar, and discuss their biostratigraphic, paleobiogeographic, and tectonic implications.
Geologic setting and stratigraphy
The brachiopod fossils for the present study were collected from the Kya-in Taung section (16°31′25″N, 97°36′51″E, also named the Tower section by Yuan et al., Reference Yuan, Aung, Henderson, Zhang, Zaw, Cai, Ding and Shen2020) in the northwestern part of the Zwekabin Range, Hpa-an Township, Kayin State, Myanmar (Figs. 1, 2). As shown in Figure 1.2, the Kya-in Taung section is located very close to the Paung Laung Mawchi Zone or the Medial-Myanmar Suture Zone (MMSZ) (Mitchell et al., Reference Mitchell, Hlaing, Htay, Mantajit and Potisat2002, Reference Mitchell, Chung, Oo, Lin and Hung2012, Reference Mitchell, Htay and Htun2015), which separates the Slate Belt to the west, dominated by the Mergui Group and its equivalents containing glacial-marine diamictites, and the Shan Plateau with late Proterozoic to Cretaceous stratigraphic successions to the east (Mitchell, Reference Mitchell2018). However, structural complexity and discontinuous outcrops have hampered a full understanding of the MMSZ and thus its position is uncertain in central and eastern Myanmar, especially in the Moulmein and Mawchi areas (Ridd, Reference Ridd, Barber, Zaw and Crow2017). That is, whether the Moulmein and Mawchi areas belong to the Slate Belt or the Shan Plateau remains in debate owing to their proximity to the MMSZ and the lack of diamictite in the Taungnyo Group and Mawchi series. In this paper, the diamictite interval of the Taungnyo Group (16°23′20″N, 97°40′29″E) is reported from the Taungnyo Range for the first time, ~10 km south of Moulmein and 50 km south of the studied section (Fig. 1.2), which indicates that the studied section belongs to the Slate Belt. The diamictite interval is > 20 m and some pebbles are clearly observed (Fig. 1.3, 1.4). Similarly, diamictites are also found in a stream near the Kan Zan Mine, Sibu village, Mawchi area (approximately 19°30′45″N, 96°48′29″E). Therefore, the Moulmein and Mawchi areas are considered to be incorporated into the Slate Belt instead of the Shan Plateau.
As mentioned above, the strata in the studied area have been historically assigned to the Taungnyo Group (Brunnschweiler, Reference Brunnschweiler1970); they mainly consist of interbedded marine sandstone, shale, siltstone, and mudstone with a total thickness of ~700 m in the Hpa-an area, with the topmost 30 m of this group dominated by well-bedded, yellow, richly fossiliferous mudstone or fine-grained sandstone with diverse brachiopod fossils (Fig. 2.2, 2.3). The overlying Moulmein Limestone is composed of massive limestone (Fig. 2.5) with reddish sandstone and marls grading upward to patch-reef facies (Brunnschweiler, Reference Brunnschweiler1970; Thura Oo et al., Reference Oo, Tin and Nyunt2002). Although the boundary between the Taungnyo Group and the Moulmein Limestone at Point 1156 (probably the Kya-in Taung section in this paper) is nearly concordant, Brunnschweiler (Reference Brunnschweiler1970) still suggested the presence of an angular unconformity, mainly based on the differences of strata attitude between peak and hillside and paleontological ages between the strata. However, our recent investigation shows that the boundary interval spanning the two groups in the current section is characterized by transitional beds from mudstone to carbonates with a continuous late Kungurian conodont fauna (Yuan et al., Reference Yuan, Aung, Henderson, Zhang, Zaw, Cai, Ding and Shen2020) and no obvious physical or stratigraphic break (Figs. 2.4, 3).
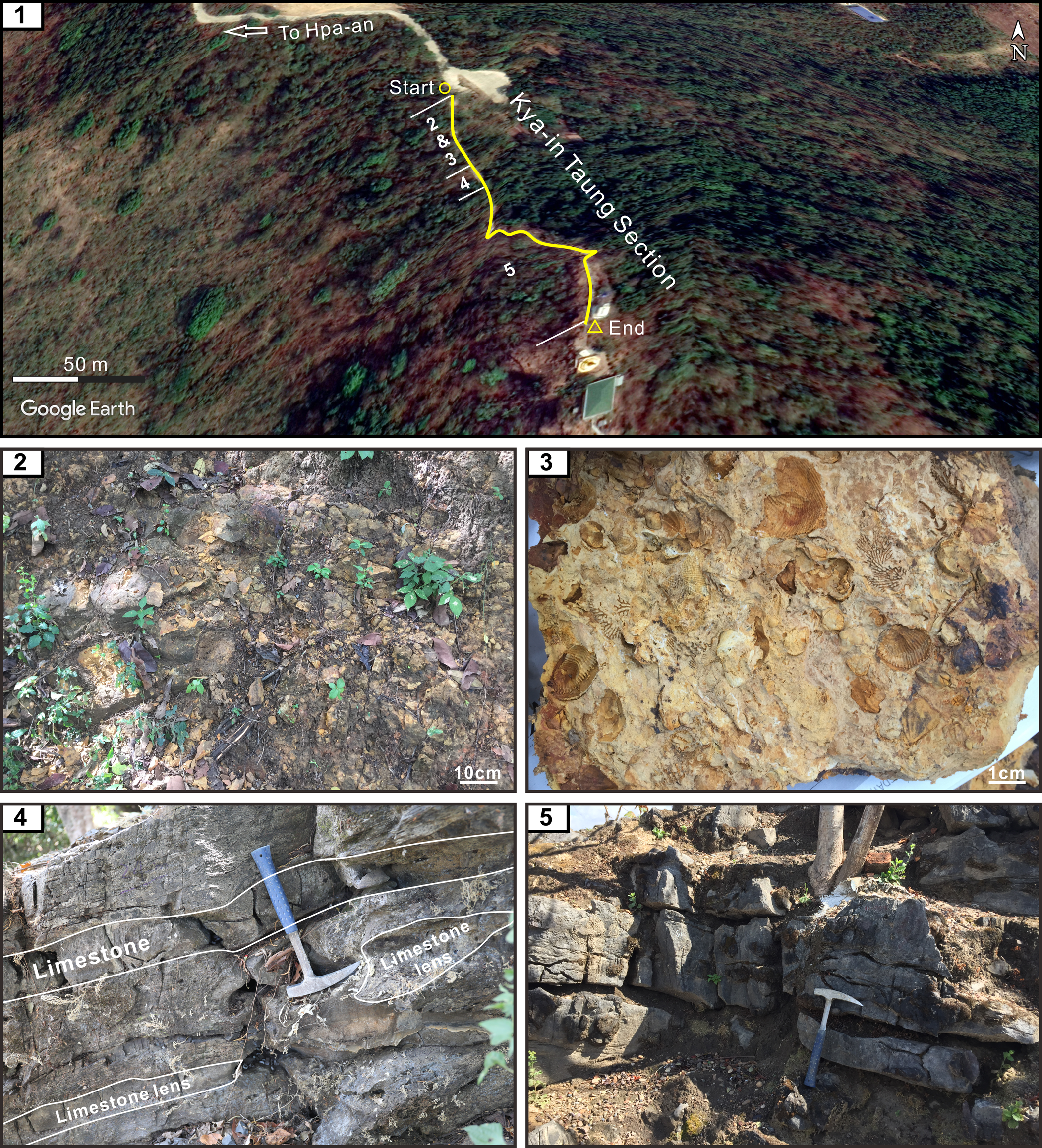
Figure 2. (1) Google map of the Kya-in Taung section; numbers 2, 3, 4, and 5 represent the rock horizons in the following: (2) yellowish mudstone of the Taungnyo Group; (3) diverse brachiopod fossils in the mudstone; (4) transitional beds from mudstone to limestone; (5) massive limestone with chert nodules.
Similar stratigraphic successions are well developed in the Phuket Terrane in southern Thailand, in the Thailand segment of the Karen-Tenasserim Unit. There, the siliciclastic Kaeng Krachan Group is conformably overlain by the carbonate Ratburi Group, which mirrors the Taungnyo Group and the overlying Moulmein Limestone in the Moulmein area, southern Myanmar (Ridd, Reference Ridd2009; Ueno and Charoentitirat, Reference Ueno, Charoentitirat, Ridd, Barber and Crow2011; Ridd and Watkinson, Reference Ridd and Watkinson2013; Yuan et al., Reference Yuan, Aung, Henderson, Zhang, Zaw, Cai, Ding and Shen2020). In addition, the fossil records and ages are also strongly correlative, e.g., the similar Spinomartinia prolifica-dominated fauna reported from the topmost layer of the Kaeng Krachan Group (Waterhouse, Reference Waterhouse1981; Shi et al., Reference Shi, Raksaskulwong, Campbell, Hill, Henderson and Bamber2002) and the late Kungurian radiolarian Pseudoalbaillella Holdsworth and Jones, Reference Holdsworth and Jones1980 from the basal Ratburi Group (Thassanapak et al., Reference Thassanapak, Udchachon, Chareonmit and Burrett2020).
In short, the Slate Belt of Myanmar to which the studied area belongs, together with the Phuket Terrane of Thailand, constitute the Phuket-Slate Belt Terrane of Ridd and Watkinson (Reference Ridd and Watkinson2013), which is basically equivalent to the Karen-Tenasserim Unit of Bender (Reference Bender1983) although different views exist in terms of the bounding faults of these two blocks (Ridd, Reference Ridd, Barber, Zaw and Crow2017) (Fig. 1.1). On a broader tectonic scale, the Karen-Tenasserim Unit is considered a part of the Irrawaddy Block that is separated from the Sibuma Block by the MMSZ and the Three Pagodas Fault belt in the south (Ridd, Reference Ridd2016; Ridd et al., Reference Ridd, Crow and Morley2019).
Materials and methods
The uppermost part of the Taungnyo Group in the Kya-in Taung section is dominated by the massive mudstone interbedded with several thin limestone beds. Brachiopod samples were collected from > 40 mudstone layers and are grouped into eight units (Fig. 3). The specimens are almost entirely internal and external molds except for a very few body fossils. They were repaired using a graver and a pneumatic sharpening pen under a microscope, coated with smoked magnesium, and photographed under Nikon KEYENCE D3X and Nikon SMZ25 stereomicroscopes. In addition, dozens of latex casts were made to observe the complex spinosity and internal structures of the specimens.

Figure 3. Stratigraphic succession of the Kya-in Taung section with the brachiopod and conodont fossils; conodont data from Yuan et al. (Reference Yuan, Aung, Henderson, Zhang, Zaw, Cai, Ding and Shen2020). Color of the lithology approximately represents the Munsell color of rocks measured in the field.
Repositories and institutional abbreviations
The specimens described herein are housed in the Nanjing Institute of Geology and Palaeontology (NIGP), Chinese Academy of Sciences, Nanjing, China. Other institutional abbreviations used in the text are: PIN = Palaeontological Institute, Akademia Nauka, Moscow, Russia; TBR = Geological Survey of Thailand, Department of Mines, Bangkok; UL = Univerza v Ljubljani, Ljubljana, Slovenia; USNM = National Museum of Natural History (U.S. National Museum), Smithsonian Institution, Washington, DC; YNGS = Yunnan Institute of Geological Sciences, Kumming, Yunnan, China.
Results
Brachiopod fauna and its age determination
In total, 23 brachiopod species in 23 genera are found in the topmost part of the Taungnyo Group at the Kya-in Taung section. Spinomartinia prolifica Waterhouse, Reference Waterhouse1981 is the most dominant species in the brachiopod fauna. Also very abundant are Retimarginifera alata Waterhouse, Reference Waterhouse1981, Kutorginella paucispinosa Waterhouse, Reference Waterhouse1981, Karavankina typica Ramovš, Reference Ramovš1969, Stenoscisma quasimutabilis Waterhouse, Reference Waterhouse1981, and Spiriferella modesta Waterhouse, Reference Waterhouse1981. These dominant species are all shared with those from the upper Kaeng Krachan Group at Ko Yao Noi in southern Thailand (Waterhouse, Reference Waterhouse1981). Other shared, albeit less common, species include Chonetinella cymatilis Grant, Reference Grant1976, Vediproductus dissimilis (Waterhouse, Reference Waterhouse1981), Derbyia perplexus (Waterhouse, Reference Waterhouse1981), Kasetia kaseti Waterhouse, Reference Waterhouse1981, and Yaonoiella mantajiti Waterhouse, Reference Waterhouse1983b. The strong similarity in species composition between the present brachiopod fauna and that of the Ko Yao Noi Formation clearly implies an age equivalence between the two faunas. Waterhouse (Reference Waterhouse1981) initially considered the age of the Spinomartinia prolifica-dominated brachiopod fauna from Ko Yao Noi to be Sakmarian, but the age was later revised by Archbold (Reference Archbold1999) to late Artinskian based on comparisons with the faunas of Western Australia, or even as late as early Kungurian (J.M. Dickins and G.R. Shi, personal communication, as cited by Shi et al., Reference Shi, Raksaskulwong, Campbell, Hill, Henderson and Bamber2002). These different views highlight the fact that the age of the Spinomartinia prolifica brachiopod remains unclear.
Among the brachiopods described here, Chonetina cf. Chonetina artiensis (Krotow, Reference Krotow1885) most closely resembles Chonetina artiensis (Krotow, Reference Krotow1885) recorded from the Artinskian of central Urals, Russia; it is also relatively similar to Chonetina sp. indet. from the Wordian of southern Thailand (Grant, Reference Grant1976). Chonetinella cymatilis Grant, Reference Grant1976 was first reported from the Ratburi Group of Wordian age in southern Thailand, and Chonetinella andamanensis Waterhouse, Reference Waterhouse1981 from the upper Kaeng Krachan Group is suggested to be included in the former species according to their minor undistinguished differences. Paramesolobus ivanovae Afanas'eva, Reference Afanas'eva1975 is one of the two species of this rare genus and is commonly limited to a Kasimovian age of late Carboniferous (Afanas'eva, Reference Afanas'eva1975). Additionally, the other closely related species, Paramesolobus sinuosus (Schellwien, Reference Schellwien1900), was once reported from the Lashkargaz Formation of Kungurian age in the Karakorum Range, Pakistan (Angiolini, Reference Angiolini1996), which confirms the age extension of this genus to the late Cisuralian. Karavankina typica was first discovered from the Kazanian of northern Yugoslavia (now assigned to the Roadian). Vediproductus dissimilis is highly similar to Vediproductus punctatiformis (Chao, Reference Chao1927), which is the nominate species of the Permocryptospirifer-Vediproductus punctatiformis Biozone of late Kungurian to Roadian age in South China (Shen, Reference Shen2018; Shen et al., Reference Shen, Zhang, Zhang, Yuan, Chen, He, Mu, Lin, Wang, Chen, Wu, Cao, Wang and Wang2019). Cimmeriella mucronata (Fang, Reference Fang1994) was widespread on the Cimmerian continents, e.g., the Yunzhug and Wululong formations of the Lhasa Block and the upper Dingjiazhai Formation in the Baoshan Block, indicating a Sakmarian to Kungurian age (Jin and Sun, Reference Jin and Sun1981; Fang and Fan, Reference Fang and Fan1994; Shen et al., Reference Shen, Shi and Zhu2000; Zhan et al., Reference Zhan, Yao, Ji and Wu2007). Kasetia kaseti was commonly recorded in Southeast Asia including the Singa Formation of Langkawi Island, northwestern Malaysia, and the upper Khao Phra Formation in central and northern Thailand (Shi et al., Reference Shi, Leman Mohd, Tan, Dheeradilok, Hinthong, Chadomrong, Putthapiban, Tansathien, Utha-aroon, Sattyarak, Nuchanong and Techawan1997, Reference Shi, Raksaskulwong, Campbell, Hill, Henderson and Bamber2002). Reticulariina atava Chronic in Newell et al., Reference Newell, Chronic and Roberts1949 was only recorded from the Copacabana Group (early Cisuralian) of south-central Peru (Newell et al., Reference Newell, Chronic and Roberts1949).
Most of the indeterminate species in this fauna are cosmopolitan elements spanning the entire Permian except for Comuquia sp. indet. and Magniplicatina sp. indet. that can provide some broad age implications. Comuquia Grant, Reference Grant1976 was usually distributed in the peri-Gondawanan region with a Sakmarian-Wordian age (e.g., Grant, Reference Grant1976; Jin and Sun, Reference Jin and Sun1981; Archbold, Reference Archbold1984; Sun, Reference Sun, Sun and Xu1991). Magniplicatina Waterhouse, Reference Waterhouse1983b was erected based on the Kungurian specimens from the Bowen Basin of eastern Australia and generally ranged from the Artinskian to the Wuchiapingian (Waterhouse and Briggs, Reference Waterhouse and Briggs1986; Briggs, Reference Briggs1998; Shen et al., Reference Shen, Shi and Archbold2003).
In short, all of the species contained in the present brachiopod fauna are generally restricted to an age from the Sakmarian to the Wordian. A further and more precise age determination is difficult if assessed merely based on the brachiopods alone. Fortunately, however, a modest but significant conodont fauna has been found associated with the brachiopod assemblage, including Mesogondolella idahoensis (Youngquist, Hawley, and Miller Reference Youngquist, Hawley and Miller1951) and Vjalovognathus nicolli Yuan et al., Reference Yuan, Zhang, Shen, Henderson, Zhang, Zhu, An and Feng2016, both indicating an unequivocal late Kungurian age (Yuan et al., Reference Yuan, Aung, Henderson, Zhang, Zaw, Cai, Ding and Shen2020).
Discussion
Biostratigraphic implications
The Spinomartinia prolifica faunas or assemblage have been widely reported from the Sibumasu Block (or Shan-Thai Terrane) and are generally regarded as a typical late Sakmarian brachiopod fauna (e.g., Shi and Waterhouse, Reference Shi and Waterhouse1991; Shi and Archbold, Reference Shi and Archbold1995; Shi et al., Reference Shi, Leman Mohd, Tan, Dheeradilok, Hinthong, Chadomrong, Putthapiban, Tansathien, Utha-aroon, Sattyarak, Nuchanong and Techawan1997, 2002; Thura Oo et al., Reference Oo, Tin and Nyunt2002; Zaw Win, Reference Win2009; Shen et al., Reference Shen, Zhang, Shi, Li, Xie, Mu and Fan2013; Mitchell, Reference Mitchell2018). However, as discussed above, the age of the well-known brachiopod fauna in this paper is much younger, at late Kungurian according to the associated conodonts uncovered from the uppermost part of the Taungnyo Group in eastern Myanmar (Yuan et al., Reference Yuan, Aung, Henderson, Zhang, Zaw, Cai, Ding and Shen2020). Interestingly, a similar brachiopod fauna has also been reported from the upper Kaeng Krachan Group in southern Thailand, where the top of this group is constrained as Kungurian by the ammonoid Agathiceras Gemmellaro, Reference Gemmellaro1887 fauna (Fujikawa et al., Reference Fujikawa, Ueno, Sardsud, Saengsrichan, Kamata and Hisada2005) and by the radiolarian Pseudoalbaillella fauna in the base of the overlying Ratburi Group (Thassanapak et al., Reference Thassanapak, Udchachon, Chareonmit and Burrett2020). Thus, it is now necessary to make comprehensive comparisons and biostratigraphic updates of the Cisuralian brachiopod faunas in the Sibumasu Block.
As mentioned above, > 60% of the species of the present fauna are shared with that from the upper Kaeng Krachan Group of Ko Yao Noi, southern Thailand (Waterhouse, Reference Waterhouse1981), which clearly indicates that the latter equivalent fauna is also of late Kungurian age. Here, the Spinomartinia prolifica-Retimarginifera alata (Sp-Ra) Assemblage is proposed to represent these late Kungurian brachiopod faunas, dominated by the species Kutorginella paucispinosa, Stereochia koyaoensis Waterhouse, Reference Waterhouse1981, Urushtenia arguta Grant, Reference Grant1976, Stenoscisma quasimutabilis, and Spiriferella modesta in addition to the two nominate species (Fig. 4). Similar brachiopod faunas include those reported from the Khao Phra Formation at the Khao Than area (Shi et al., Reference Shi, Raksaskulwong, Campbell, Hill, Henderson and Bamber2002) and the Yinyaw beds at the Mawchi area (Kyi Pyar Aung et al., Reference Aung, Shen and Naing2018) (Table 1).

Figure 4. Ciisuralian stratigraphic and faunal correlations among different sections in the Sibumasu Block. Species names in bold indicate dominance of species in the fauna. Color of the lithology approximately represents the Munsell color of rocks measured in the field. Species not otherwise mentioned in the text are Brachythyrina rectangulus (Kutorga, Reference Kutorga1844) and Brachythyrina gobbetti Shi and Waterhouse, Reference Shi and Waterhouse1991.
Table 1. Age revisions of the Cisuralian brachiopod faunas from the Sibumasu Block. No. 6 is omitted because it is a fusuline fauna in Figure 4.
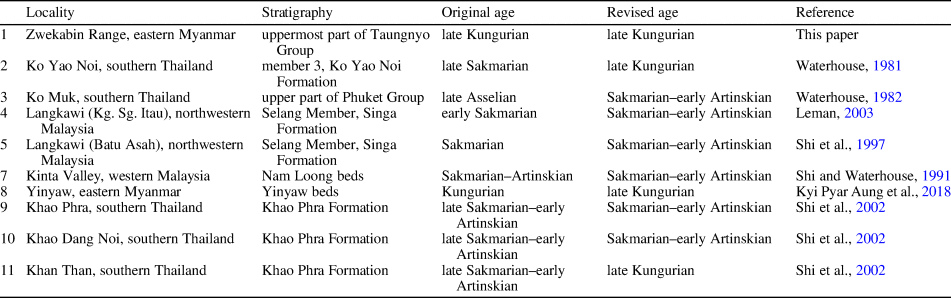
However, brachiopod faunas with different ages are also present in the Sibumasu Block. Among them, one is from the Nam Loong No. 1 Mine beds of Kinta Valley, Perak, Malaysia; its age is restricted by the Pseudofusulina kraffti (Schellwien, Reference Schellwien1909) and Misellina claudiae (Deprat, Reference Deprat1912) fusuline biozones of a late Artinskian–Kungurian age from the overlying H. S. Lee beds (Ishii, Reference Ishii1966; Shi and Waterhouse, Reference Shi and Waterhouse1991). Another is from the Selang Member of the Singa Formation at Kilim and Batu Asah of Lankawi Island (Shi et al., Reference Shi, Leman Mohd, Tan, Dheeradilok, Hinthong, Chadomrong, Putthapiban, Tansathien, Utha-aroon, Sattyarak, Nuchanong and Techawan1997); its age is constrained by the co-existence of the late Sakmarian ammonoid Metalegoceras sp. indet. (Leonova et al., Reference Leonova, Leman and Shi1999). Although both of these faunas are dominated by Spinomartinia prolifica, other abundant species vary greatly. Retimarginifera alata, Kutorginella paucispinosa, Stereochia koyaoensis, and Spiriferella modesta are extremely rich in the Sp-Ra Assemblage, but are totally absent in the two Malaysian faunas, which instead commonly contain Bandoproductus monticulus (Waterhouse, Reference Waterhouse1982), Sulciplica thailandica (Hamada, Reference Hamada1960), and Spirelytha petaliformis (Pavlova in Grunt and Dmitriev, Reference Grunt and Dmitriev1973); these elements are barely found in Sp-Ra Assemblage (Fig. 4). Therefore, it seems evident that the two Malaysian brachiopod assemblages represent a different brachiopod fauna that should be named and considered separately from the Sp-Ra Assemblage. Here, we propose the Bandoproductus monticulus-Spirelytha petaliformis (Bm-Sp) Assemblage to represent the Malaysian faunas and ascribe it to the Sakmarian to probably early Artinskian age in view of its associated late Sakmarian ammonoid and overlying late Artinskian to Kungurian fusulines. Moreover, a brachiopod fauna from the Kampung Sungai Itau section in Langkawi Island (Leman, Reference Leman2003), another fauna dominated by Bandoproductus monticulus and Sulciplica thailandica from the upper Phuket Group at the Ko Muk area (Waterhouse, Reference Waterhouse1982), and two other faunas from Khao Phra and Khao Dang Noi in central and Peninsular Thailand (Shi et al., Reference Shi, Raksaskulwong, Campbell, Hill, Henderson and Bamber2002) are suggested to be contained in this Bm-Sp Assemblage (Fig. 4; Table 1).
In summary, the previous brachiopod faunas dominated by Spinomartinia prolifica in the Sibumasu Block can be subdivided into two separate stratigraphic assemblages, i.e., the Sakmarian-early Artinskian Bm-Sp Assemblage and the late Kungurian Sp-Ra Assemblage (Fig. 4).
Paleobiogeographic and tectonic implications
As discussed above, the Cisuralian brachiopod faunas in the Sibumasu Block are generally classified into two assemblages. The older Bm-Sp Assemblage is characterized by genera with Gondwanan affinities. Bandoproductus Jin and Sun, Reference Jin and Sun1981 was only reported from the early Cisuralian of Gondwana and its surrounding area, e.g., the Lhasa Block (Jin and Sun, Reference Jin and Sun1981; Zhan et al., Reference Zhan, Yao, Ji and Wu2007), Baoshan Block (Shen et al., Reference Shen, Shi and Zhu2000), Karakorum Block (Angiolini et al., Reference Angiolini, Brunton and Gaetani2005), and Australia Plate (Briggs, Reference Briggs1998). Spirelytha Fredericks, Reference Fredericks1924, a typical bipolar genus, is mainly distributed in the Gondwanan and Boreal realms (e.g., Archbold et al., Reference Archbold, Thomas and Skwarko1993; Shi and Waterhouse, Reference Shi and Waterhouse1996; Shen et al., Reference Shen, Shi and Zhu2000, Reference Shen, Archbold, Shi and Chen2001). Sulciplica Waterhouse, Reference Waterhouse1968 has only been recorded from the Lhasa Block (Zhan et al., Reference Zhan, Yao, Ji and Wu2007) and Australia Plate (Waterhouse, Reference Waterhouse1968, Reference Waterhouse1987). Additionally, the Bm-Sp Assemblage was all obtained from the horizons of pebbly mudstone or diamictite (Fig. 4), which have widely been interpreted as glacial-marine diamictites (Stauffer and Lee, Reference Stauffer and Lee1986; Ampaiwan et al., Reference Ampaiwan, Hisada and Charusiri2009; Ridd, Reference Ridd2009; Metcalfe, Reference Metcalfe2013). Thus, the Bm-Sp Assemblage can be regarded as a typical cold-water fauna in terms of its Gondwanan-type genera and associated ice-rafted marine sediment. In comparison, the younger Sp-Ra Assemblage shows a somewhat different faunal composition. The genera with Gondwanan affinities are conspicuously diminished with the few exceptions of Retimarginifera Waterhouse, Reference Waterhouse1970, Costatumulus Waterhouse, Reference Waterhouse1983a, Neospirifer Fredericks, Reference Fredericks1924, and Spinomartinia Waterhouse, Reference Waterhouse1968; those with Cathaysian affinities, e.g., Vediproductus Sarycheva in Ruzhentsev and Sarycheva, Reference Ruzhentsev and Sarycheva1965, Brachythyrina Fredericks, Reference Fredericks1929, and Urushtenia Licharew, Reference Licharew1935, are increased. Furthermore, the genera mainly restricted to Cimmerian continents—including Stereochia Grant, Reference Grant1976, Kasetia Waterhouse, Reference Waterhouse1981, and Cimmeriella Archbold and Hogeboom, Reference Archbold and Hogeboom2000—occupy a large proportion of this assemblage. Moreover, the Sp-Ra Assemblage was mainly recorded in the mudstones without glacial deposits and much closer to the base of carbonates (Fig. 4). In short, the Sp-Ra Assemblage can be best regarded as a cold-temperate fauna with an admixture of both Cathaysian and Gondwanan taxa.
Interestingly, the spatial distribution pattern of these two separate brachiopod assemblages varies distinctly. The Sakmarian to early Artinskian cold-water Bm-Sp Assemblage in the glacial-marine diamictites was widely distributed in the Sibumasu Block including both Irrawaddy and Simuma blocks. This case prominently indicates that the Irrawaddy and Sibuma blocks were both proximal and attached to the northern margin of Gondwana, and thus influenced by its large continental ice sheet during the Sakmarian and early Artinskian (Fig. 5.1, 5.2). However, the cold-temperate mixed Sp-Ra Assemblage with a late Kungurian age has only been formally reported from the Irrawaddy Block, with as yet no evidence confirmed from the Sibuma Block (Fig. 5.1, 5.3). Coincidentally, the fusuline Pseudofusulina-Praeskinnerella (Ps-Pr) Assemblage with a late Artinskian to early Kungurian age was only recorded in the Sibuma Block (Fig. 5.1, 5.3) and shows faunal affinities with the paleotropical Tethyan fusuline faunas. This assemblage includes a fusuline fauna containing Pseudofusulina Dunbar and Skinner, Reference Dunbar and Skinner1931 and ?Praeskinnerella Bensh, Reference Bensh1991 from the Yala area of southernmost Thailand (Ueno et al., Reference Ueno, Arita, Meno, Sardsud and Saesaengseerung2015) as well as another consisting of Pseudofusulina kraffti and Misellina claudiae from the Kinta Valley of western Malaysia (Ishii, Reference Ishii1966) (Fig. 4).

Figure 5. (1) Distribution of Irrawaddy and Sibuma blocks in Southeast Asia, modified from Ridd (Reference Ridd2016), with 11 brachiopod and fusuline localities; Arabic numerals refer to Table 1. (2, 3) Paleogeographic maps of the Tethyan area during the Sakmarian and Kungurian, respectively, showing the distribution of brachiopod and fusuline faunas; base map courtesy of Christopher Scotese. Geographical and paleogeographic distributions of the brachiopod faunas from the previous Sibumasu Block. AR = Arabian Plate; AS = Australia Plate; BJ = Borneo and Java; BS = Baoshan Block; H = Tethys Himalaya; IC = Indochina; ID = Indian Plate; IR = Iran; IW = Irrawaddy Block; LS = Lhasa Block; SC = South China; SI = Sibuma Block; SQ = South Qiangtang Block; TC = Tengchong Block.
In short, the nature and sequence of faunal changes between the Irrawaddy and Sibuma blocks varied greatly during the Cisuralian, with the former changing from a cold-water dominated fauna to a cold-temperate mixed fauna and the latter transformed from cold-water fauna directly to a warm-water affiliated fauna without going through a mixed or transitional faunal appearance. The cause of cold to mixed brachiopod faunal change in the Irrawaddy Block is likely related to the interplay of the northward drifting eastern Cimmerian continents from the peri-Gondwanan region and contemporaneous global climatic amelioration following the retreat of the main Permian Gondwanan glaciation episode in the earliest Permian (Shi and Archbold, Reference Shi and Archbold1995, Reference Shi, Archbold, Hall and Holloway1998; Haig et al., Reference Haig, Mory, McCartain, Backhouse, Håkansson, Ernst, Nicoll, Shi, Bevan, Davydov, Hunter, Keep, Martin, Peyrot, Kossavaya and Santos2017). On the other hand, the direct shift from a cold-water fauna to a warm-water affiliated fauna experienced by the Sibuma Block is more puzzling. Not only did this demonstrate a clear departure from the Irrawaddy Block in terms of their contemporaneous biogeographic affinities during the Artinskian-Kungurian, it also points to some significant differences in paleogeographic and paleoclimatic conditions experienced by the two blocks during this time. One mechanism to account for both of these is that, during the Artinskian–Kungurian, the two blocks underwent different tectonic evolution pathways, rapidly diverging from one another while both were still subject to background global warming (as a consequence of diminishing deglaciations in Gondwana). During this process, it is likely that the Sibuma Block separated from and drifted north of the Irrawaddy Block. While both blocks were drifting north, progressively moving into warmer climatic zones, the Sibuma Block was probably drifting at a faster rate allowing it to reach and merge with the warm-temperate zone much earlier than the Irrawaddy Block from which it would have created considerable latitudinal distance/separation by the late Kungurian (Fig. 5.3).
There is potential to apply the above interpretation more broadly across the Cimmerian continents in South and Southeast Asia. For example, the Lhasa Block, like the Irrawaddy Block, is characterized by mixed conodonts and relatively cool-water brachiopods (Yuan et al., Reference Yuan, Zhang, Shen, Henderson, Zhang, Zhu, An and Feng2016, Reference Yuan, Aung, Henderson, Zhang, Zaw, Cai, Ding and Shen2020; Xu et al., Reference Xu, Zhang, Qiao and Shen2019), whereas the South Qiangtang Block is dominated by brachiopod and fusuline faunas with typical Cathaysian affinities (Zhang et al., Reference Zhang, Wang, Zhang and Yuan2012; Shen et al., Reference Shen, Sun, Zhang and Yuan2016), like those of the Sibuma Block. If the aforementioned interpretative model accounting for the late Cisuralian biogeographic difference between the Irrawaddy and Sibuma blocks is followed here, the Mesotethys (also known as the Bangong-Nujiang Ocean in some literature) separating the South Qiangtang Block in the north from the Lhasa Block in the south would have opened up before the Guadalupian (middle Permian), and this is certainly consistent with known stratigraphic and paleontologic evidence (Zhang et al., Reference Zhang, Zhang, Yuan, Xu and Qiao2019, Reference Zhang, Aung, Shen, Zhang, Zaw, Ding, Cai and Sein2020). Further, it is worth noting that the Tibetan Mesotethys is considered to have extended southward to southeastward into Southeast Asia by the end of Cisuralian, where it would have forced disintegration of the Sibumasu Block into the Sibuma Block in the north and the Irrawaddy Block in the south.
Conclusions
A new brachiopod fauna is described from the uppermost part of the Taungnyo Group at the Kya-in Taung section in the Zwekabin Range, eastern Myanmar, with significant implications for biostratigraphy, paleobiogeography, and tectonics, as summarized below.
(1) The new brachiopod fauna dominated by Spinomartinia prolifica from eastern Myanmar is of late Kungurian age and contains abundant Retimarginifera alata, Kutorginella paucispinosa, Karavankina typica, Stenoscisma quasimutabilis, and Spiriferella modesta in addition to the nominate species.
(2) Based on comprehensive comparisons of the Cisuralian brachiopod faunas and other data in the Sibumasu Block, the previous brachiopod faunas dominated by Spinomartinia prolifica are subdivided into two stratigraphic assemblages, the older (Sakmarian–early Artinskian) Bandoproductus monticulus-Spirelytha petaliformis (Bm-Sp) Assemblage and the younger (late Kungurian) Spinomartinia prolifica-Retimarginifera alata (Sp-Ra) Assemblage.
(3) The Bm-Sp Assemblage, a typical cold-water brachiopod fauna, is widely distributed in the Sibumasu Block, including both the Irrawaddy and Sibuma blocks, whereas the mixed Sp-Ra Assemblage is only recorded in the Irrawaddy Block, and the Sibuma Block contains paleotropical Tethyan fusuline faunas from the Kungurian.
(4) Using paleobiogeographic evidence, the Sibumasu Block is interpreted to have likely disintegrated into the Irrawaddy Block in the south and the Sibuma Block in the north by the end of Cisuralian, separated by the Thai-Myanmar Mesotethys.
Systematic paleontology
The systematic study above the genus level largely follows classifications proposed by Brunton et al. (Reference Brunton, Lazarev, Grant, Jin and Kaesler2000) for Productida, Williams et al. (Reference Williams, Brunton, Wright and Kaesler2000) for Orthotetida, Savage et al. (Reference Savage, Manceñido, Owen and Kaesler2002) for Rhynchonellida, Alvarez and Rong (Reference Alvarez, Rong and Kaesler2002) for Athyridida, Carter et al. (Reference Carter, Johnson, Gourvennec, Hou and Kaesler2006) for Spiriferida, and Carter and Johnson (Reference Carter, Johnson and Kaesler2006) for Spiriferinida.
Order Productida Sarycheva and Sokolskaya, Reference Sarycheva and Sokolskaya1959
Family Anopliidae Muir-Wood, Reference Muir-Wood1962
Genus Chonetina Krotow, Reference Krotow1888
Type species
Chonetella artiensis Krotow, Reference Krotow1885 from the Artinskian of the Urals, Russia.
Chonetina cf. Chonetina artiensis (Krotow, Reference Krotow1885)
Figure 6.1–6.4
Occurrence
Units 1, 5, and 6, Taungnyo Group, Zwekabin Range, Myanmar.

Figure 6. (1–4) Chonetina cf. Chonetina artiensis (Krotow, Reference Krotow1885), internal molds of ventral valve, NIGP 173929–173932. (5–9) Chonetinella cymatilis Grant, Reference Grant1976: (5) internal mold of ventral valve, NIGP 173933; (6–8) external molds of dorsal valve, NIGP 173934–173936; (9) internal mold of ventral valve, NIGP 173937. (10–16) Paramesolobus ivanovae Afanas'eva, Reference Afanas'eva1975: (10) internal mold of ventral valve, NIGP 173938; (11) external mold of dorsal valve partly covered by broken internal mold of ventral valve, NIGP 173939; (12–16) external molds of dorsal valve, NIGP 173940–173944. (17–21) Comuquia sp. indet.: (17–20) external molds of dorsal valve, NIGP 173945–173948; (21) internal mold of ventral valve, NIGP 173949. (22–28) Retimarginifera alata Waterhouse, Reference Waterhouse1981: (22) external mold of dorsal valve partly covered by internal mold of ventral valve, NIGP 173950; (23–27) external molds of dorsal valve, NIGP 173951–173955; (28) latex cast of internal mold of dorsal valve resting inside ventral valve, NIGP 173956. Scale bars = 5 mm.
Description
Ventral valve small, 6.3–8.9 mm long, 9.1–11.2 mm wide, strongly convex, moderately geniculate, semicircular in outline, widest at hinge or slightly anterior to hinge; cardinal extremities blunt, near right angle; beak small, pointed, strongly incurved, distinctly projecting beyond hinge line, with umbonal slopes sharply inclined; ears flat, very small; linear sulcus originating from umbo, very narrow, deep; costellae relatively dense but coarse, intercalating from anterior quarter of valve length; spine bases numerous, spaced in rows along costellae.
Materials
Registered specimens: four internal molds of ventral valve (NIGP 173929–173932).
Remarks
The present specimens are characterized by their small size, strongly convex ventral valve, deep ventral sulcus, costellate surface, and developed spines, which match well with those features in Chonetina. This species most resembles Chonetina artiensis (Krotow, Reference Krotow1885, pl. 4, figs. 16–18) in size, outline, and its strongly overhanging beak, but is slightly distinguished by its narrower apical angle and smaller ears. Another species, Chonetina sp. indet., from the Wordian of southern Thailand (Grant, Reference Grant1976, pl. 12, figs. 1–19, text-fig. 11) is similar to the present species but differs in its much stronger costellation and much larger ears. Our specimens can be easily differentiated from most species of Chonetina (e.g., Chonetina dalmiriensis Reed, Reference Reed1944 and Chonetina noenygaardi Dunbar, Reference Dunbar1955) by their strongly incurved beaks that obviously overhang the hinge line.
Family Rugosochonetidae Muir-Wood, Reference Muir-Wood1962
Genus Chonetinella Ramsbottom, Reference Ramsbottom1952
Type species
Chonetes flemingi Norwood and Pratten, Reference Norwood and Pratten1855 from the Pennsylvanian of Texas, USA.
Chonetinella cymatilis Grant, Reference Grant1976
Figure 6.5–6.9
- Reference Grant1976
Chonetinella cymatilis Grant, p. 77, pl. 16, figs. 1–58.
- Reference Waterhouse1981
Chonetinella andamanensis Waterhouse, p. 65, pl. 2, figs. 18, 19, pl. 3, figs. 1–18.
Holotype
USNM 211993, Ratiburi Group, Ko Muk, southern Thailand (Grant, Reference Grant1976, pl. 16, figs. 26–30).
Occurrence
Units 6 and 7, Taungnyo Group, Zwekabin Range, Myanmar; Ko Yao Noi Formation, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand; Ratiburi Group, Ko Muk, southern Thailand.
Description
Shell 7.5–9.5 mm long, 16.2–19.1 mm wide; outline transverse, inverted trapezoidal; greatest width at hinge; cardinal extremities slightly extended. Ventral valve moderately convex in lateral profile; interarea very low; beak short; ears small, flat to slightly convex; sulcus beginning from umbo, becoming very wide and deep anteriorly. Dorsal valve slightly to moderately concave; fold commencing approximately at one-third valve length anterior to dorsal beak, relatively high and wide; capillae very dense, ~20 per 5 mm anteriorly, generally bifurcating approximately at midvalve and near anterior margin; growth lines distinct, not very regularly distributed.
Materials
Registered specimens: two internal molds of ventral valve and three external molds of dorsal valve (NIGP 173933–173937).
Remarks
The external appearance of the available external molds of the dorsal valve is perfectly consistent with that of the type specimens of Chonetinella cymatilis, e.g., sulcus deepening and widening anteriorly, proportionately greater width, and weak costellae. In addition, the specimens from the Ko Yao Noi Formation of southern Thailand, named as Chonetinella andamanensis, are better assigned to the present species. The minor differences (e.g., less transverse outline, more alate cardinal extremities) compared with Chonetinella cymatilis can be treated as intraspecific varation because they are even variable in the studied specimens of Waterhouse (Reference Waterhouse1981).
Genus Paramesolobus Afanas'eva, Reference Afanas'eva1975
Type species
Paramesolobus ivanovae Afanas'eva, Reference Afanas'eva1975 from the Kasimovian of Russia.
Remarks
Paramesolobus can be readily distinguished from Mesolobus Dunbar and Condra, Reference Dunbar and Condra1932 by its capillate external ornament and absence of lamellae. This genus is closely similar to Capillomesolobus Pečar, Reference Pečar1986, but slightly differs by its larger size, coarser bifurcating costellation, and more frequently without a ventral median lobe (Pečar, Reference Pečar1986). This genus is also similar to Chonetinella in general appearance, but normally has a ventral sulcus bearing a median fold and a dorsal fold bearing a median sulcus, and no concentric wrinkles.
Paramesolobus ivanovae Afanas'eva, Reference Afanas'eva1975
Figure 6.10–6.16
- Reference Afanas'eva1975
Paramesolobus ivanovae Afanas'eva, p. 102, fig. 4.
Holotype
PIN 132/2942, Kasimovian, Russia (Afanas'eva, Reference Afanas'eva1975, fig. 4).
Occurrence
Units 2, 6, and 8, Taungnyo Group, Zwekabin Range, Myanmar; Khamovnicheskiy Horizon, Moscow Basin, Russia.
Description
Shell 8.1–10.5 mm long, 14.1–17.7 mm wide, semicircular to transversely subrectangular in outline, with greatest width mostly at hinge; cardinal extremities obtuse, ~80°. Ventral valve moderately convex in lateral profile; beak small, blunt; ears small, flat to slightly convex, not clearly demarcated from visceral region; sulcus beginning from umbo, widening and deepening anteriorly, with narrow, rounded median lobe; capillae very fine, delicate. Dorsal valve slightly to moderately concave; fold relatively low with narrow, shallow median sulcus; capillae very dense, 4–6 per mm at anterior margin, generally bifurcating approximately at midvalve. Ventral interior with median septum, extending approximately to one-third of valve length; pseudopunctae radially aligned.
Materials
Registered specimens: one internal mold of ventral valve and six external molds of dorsal valve (NIGP 173938–173944).
Remarks
These specimens can be safely assigned to the genus Paramesolobus mainly based on a median lobe in the ventral sulcus and very fine, bifurcated capillae. Paramesolobus ivanovae and Paramesolobus sinuosus are very close to each other. The maximum width of the former is at the hinge, whereas that of the latter is mostly at the shell midlength; also, the ears of the latter are well demarcated from the visceral region. Although the outline, greatest width, presence or absence of a ventral median lobe, and depth of dorsal median sulcus show some intraspecific variation in our specimens, they are better included in Paramesolobus ivanovae in view of the greatest width being mostly at the hinge and the ears being not clearly demarcated from the visceral region.
Family Productellidae Schuchert, Reference Schuchert and Pompeckj1929
Genus Comuquia Grant, Reference Grant1976
Type species
Comuquia modesta Grant, Reference Grant1976 from the Artinskian of southern Thailand.
Remarks
Comuquia shares a similar overall appearance with Costispinifera Muir-Wood and Cooper, Reference Muir-Wood and Cooper1960 and Spinomarginifera Huang, Reference Huang1932, but mainly differs in the absence of distinct ribbing or costae and more distinct spines. Echinauris Muir-Wood and Cooper, Reference Muir-Wood and Cooper1960 is also easily confused with Comuquia, however, specimens of the former normally have a smooth surface, much longer spines on the ears, and developed brachial ridges in the dorsal interior.
Comuquia sp. indet.
Figure 6.17–6.21
Occurrence
Units 1–3 and 5–7, Taungnyo Group, Zwekabin Range, Myanmar.
Description
Shell small, 9.8–10.5 mm long, 12.6–13.6 mm wide, subquadrate in outline, deeply concavo-convex in profile; greatest width generally at hinge; cardinal extremities near right angle. Ventral valve strongly convex, with maximum convexity anterior to umbonal region; beak small, pointed, strongly enrolled, slightly projecting beyond hinge line; umbonal slopes steep; ears small, triangular, slightly convex, well demarcated with visceral disc by groove; sulcus absent; costae obscure; concentric wrinkles very weak, only observed anterior to geniculation; spines in row on concentric lines and scattered on flank slopes. Dorsal valve moderately to strongly concave; umbonal region forming relatively deep concavity; ears small, flattened to slightly concave; fold absent; costae absent but wrinkles prominent; thin spines almost covering whole valve; some dimples present.
Materials
Registered specimens: one internal mold of ventral valve and four external molds of dorsal valve (NIGP 173945–173949).
Remarks
The present specimens very likely belong to Comuquia on account of their consistent size, outline, ornamentation, deeply concave dorsal valve, and especially the absence of ribbing and more distinct spines on the ventral valve. Comuquia modesta, the type species, and Comuquia himalayaensis Jin and Sun, Reference Jin and Sun1981 both have much more elongate outlines than the present species, but the former has much stronger ventral spines. Additionally, Comuquia himalayaensis exhibits slightly less-developed ventral spines. Comuquia australis Archbold, Reference Archbold1984 differs from the present species by its prominent lamellae irregularly spaced on ventral valve and absence of spines on dorsal valve. Comuquia mushirebuca Sun, Reference Sun, Sun and Xu1991 differs by its much smaller size and less curved ventral beak.
Genus Retimarginifera Waterhouse, Reference Waterhouse1970
Type species
Retimarginifera perforata Waterhouse, Reference Waterhouse1970 from the Kungurian of Western Australia.
Retimarginifera alata Waterhouse, Reference Waterhouse1981
Figure 6.22–6.28
- Reference Waterhouse1981
Retimarginifera alata Waterhouse, p. 80, pl. 11, figs. 7–16, pl. 12, figs. 1–8, pl. 13, fig. 1, pl. 14, fig. 1.
- Reference Win2009
Retimarginifera cf. Retimarginifera alata; Zaw Win, p. 148, fig. 2.4–2.6.
Holotype
TBR 82, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand (Waterhouse, Reference Waterhouse1981, pl. 12, fig. 7).
Occurrence
Units 1–3 and 5–8, Taungnyo Group, Zwekabin Range, Myanmar; Ko Yao Noi Formation, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand.
Description
Shell small, 9.2–11.1 mm long, 16.5–21.1 mm wide, transversely reverse trapezoid in outline, concavo-convex in lateral profile, widest at hinge; cardinal extremities acute, ~40°. Ventral valve moderately convex, strongly geniculated; beak incurved, slightly over hinge line; umbonal angle ~110°; umbonal slopes sharply inclined; ears apparently alate, well demarcated from visceral disc; sulcus originating anterior to umbo, slightly widening but obviously deepening and anterior to midvalve; costae slightly coarse; reticulation faintly shown in ventral internal mold; one or two rows of spines arranged in hinge. Dorsal valve deeply concave; ears small, alate; fold beginning approximately at midvalve, prominent anteriorly; costae look more delicate than those of ventral valves, 6–8 per 5 mm, crossed by relatively dense concentric wrinkles especially on posterior disc, producing delicate reticulate ornament, with some distinct concentric rugae spaced anterolaterally. Dorsal interior with strong, radial, spaced endospines.
Materials
Registered specimens: five external molds of dorsal valve, one external mold of dorsal valve resting inside internal mold of ventral valve, and latex cast of one internal mold of dorsal valve resting inside ventral valve (NIGP 173950–173956).
Remarks
The available specimens can be readily assigned to Retimarginifera based on their relatively small size in Marginiferinae, maximum width at the hinge, deep ventral sulcus, reticulate ornament on both valves, and spines arranged on the ears. This species is very close to Retimarginifera perforata according to the identical ears and similar overall appearance, but differs in its more delicate costae and wider sulcus anteriorly. Retimarginifera celeteria Grant, Reference Grant1976 from the Rat Buri Limestone of southern Thailand bears smaller ears, a narrower sulcus, and slightly finer reticulation when compared with the present species.
Family Productidae Gray, Reference Gray1840
Genus Kutorginella Ivanova, Reference Ivanova1951
Type species
Kutorginella mosquensis Ivanova, Reference Ivanova1951 from the upper Carboniferous in the Moscow Basin, Russia.
Remarks
This genus is characterized by its extended funnel-shaped or tubular trail and lateral ridges continuous with ear baffles (Ivanova, Reference Ivanova1951; Muir-Wood and Cooper, Reference Muir-Wood and Cooper1960). Furthermore, Grant (Reference Grant1976) proposed that the presence of thick, strut spines in combination with fine spines on both valves, which links all of included species of this genus, could be a diagnostic character of Kutorginella.
Kutorginella paucispinosa Waterhouse, Reference Waterhouse1981
Figures 7, 8.1–8.3
- Reference Waterhouse1981
Kutorginella paucispinosa Waterhouse, p. 77, pl. 8, figs. 15, 16, pl. 9, figs. 1–11.
- Reference Waterhouse1981
Kutorginella fraterculus Waterhouse, p. 79, pl. 9, figs. 12–14, pl. 10, figs. 1–8, pl. 11, figs. 1–6.
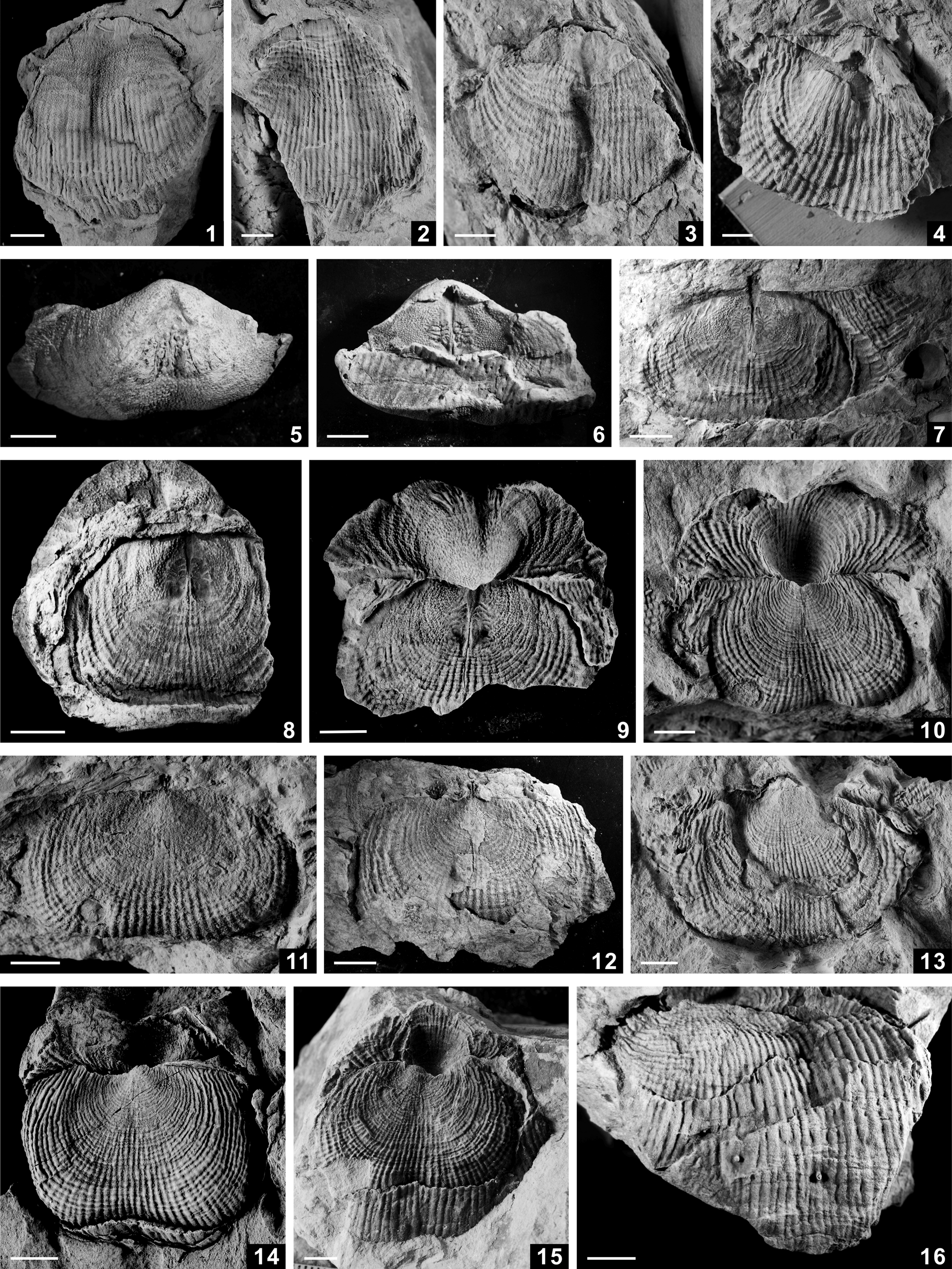
Figure 7. Kutorginella paucispinosa Waterhouse, Reference Waterhouse1981: (1, 2) internal and external molds of ventral valve, NIGP 173957; (3, 4) internal molds of ventral valve, NIGP 173958, 173959; (5, 6) internal mold of conjoined shell, NIGP 173960; (7, 8) internal molds of dorsal valve, NIGP 173961, 173962; (9, 10) internal and external molds of incomplete conjoined shell, NIGP 173963; (11–13) external molds of dorsal valve, NIGP 173964–173966; (14, 15) external molds of incomplete conjoined shell, NIGP 173967, 173968; (16) external mold of incomplete conjoined shell partly covered by ventral internal mold, NIGP 173969. Scale bars = 5 mm.

Figure 8. (1–3) Kutorginella paucispinosa Waterhouse, Reference Waterhouse1981, external molds of incomplete conjoined valve partly covered by ventral internal molds, NIGP 173970–173972. (4–14) Urushtenia arguta Grant, Reference Grant1976: (4–6) internal molds of ventral valve, NIGP 173987–173989; (7) dorsal valve interior, NIGP 173990; (8, 9) internal molds of dorsal valve, NIGP 173991, 193992; (10, 11) external molds of dorsal valve, NIGP 173993, 173994; (12, 13) external mold of ventral trail and its latex cast, NIGP 193995; (14) latex cast of ventral trail, NIGP 193996. (15–23) Karavankina typica Ramovš, Reference Ramovš1969: (15) internal mold of dorsal valve, NIGP 173973; (16, 17) external mold of dorsal valve and its latex cast, NIGP 173974; (18–20) external molds of dorsal valve, NIGP 173975–173977; (21–23) external mold of dorsal valve and details of its spinosity, NIGP 173978. Scale bars = 5 mm, unless otherwise labeled.
Holotype
TBR 58, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand (Waterhouse, Reference Waterhouse1981, pl. 9, figs. 1, 2).
Occurrence
Units 1–6 and 8, Taungnyo Group, Zwekabin Range, Myanmar; Ko Yao Noi Formation, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand.
Description
Shell small to medium, 22.5–34 mm long, 27.9–45 mm wide, transversely subrectangular in outline, maximum width at or anterior to shell midlength; cardinal extremities obtuse. Ventral valve moderately convex; beak incurved, slightly overhanging hinge line, at ~100° angle; ears large, extended, slightly convex; sulcus commencing from beak, moderately deep, slightly widening and deepening anteriorly; costae low, fairly strong, round-crested, five or six per 5 mm near anterior margin, crossed by low concentric rugae posteriorly and laterally, forming distinct reticulation; normal spines spaced over entire disc, numerous on umbonal region and ears; 1–3 different strut spines arranged somewhat in a row approximately at geniculation; spine bases ~2–3 mm in diameter observed from internal molds of ventral valve; no distinct tubular or funnel-shaped trail observed except a possibly similar short trail. Dorsal valve slightly concave to flat; strongly geniculated to form short trail anteriorly; most of ears slightly concave, separated from visceral disc; fold well-formed to relatively weak or absent; costae low with round crests, approximately seven per 5 mm anteriorly; rugae finer, approximately eight or nine per 5 mm in the front; fine, regular, reticulate ornament covering entire valve; few thin spines arranged at sides.
Ventral interior with distinct muscle marks, adductors elongated dendritic, posteriorly minor part relatively sparse but anteriorly major part very dense; diductors somewhat flabellate with deep radial ridges, distributed on both sides of adductors; close-set pits on posterior part; numerous endospines arranged anterior to geniculation. Dorsal interior with trilobed cardinal process, composed of central lobe and two lateral lobes; strong shaft forward becoming median septum, reaching approximately to three-quarters of valve length; clear dendritic adductors on both sides of median septum; lateral ridges passing across ears to form distinct ear baffles, and forward forming sharp marginal ridges at both sides, but terminating near anterior margin; brachial ridges not observed; numerous pits also posteriorly spaced.
Materials
Registered specimens: two internal molds of conjoined shell, three internal molds of ventral valve, two internal molds of dorsal valve, one external mold of ventral valve, three external molds of dorsal valve, and seven external molds of incomplete conjoined shell (four dorsal external molds partly covered by ventral internal molds) (NIGP 173957–173972).
Remarks
Waterhouse (Reference Waterhouse1981) erected two separated species of Kutorginella—Kutorginella paucispinosa Waterhouse, Reference Waterhouse1981 (pl. 8, figs. 15, 16, pl. 9, figs. 1–11) and Kutorginella fraterculus Waterhouse, Reference Waterhouse1981 (pl. 9, figs. 12–14, pl. 10, figs. 1–8, pl. 11, figs. 1–6)—based on specimens from the same layer in southern Thailand. The former species is characterized by the absence of ventral strut spines, scarce dorsal spines, and no distinct dorsal fold, whereas the latter species is distinguished by its rare ventral strut spines, numerous dorsal spines, and well-formed dorsal fold. Coincidentally, the present specimens combine the features of the two species mentioned above with the presence of rare ventral strut spines, scarce dorsal spines, and a distinct to absent dorsal fold. Moreover, all of the specimens from southern Thailand and eastern Myanmar share similar features, e.g., consistent size (30–45 mm wide), a deep sulcus, and no clear funnel-shaped trail; other features vary inconspicuously. Thus, the number of spines on each valve is probably intraspecific variation and all of those specimens are better ascribed to Kutorginella paucispinosa. The diagnostic features of this species are redefined as rare ventral strut spines, a deep persistent ventral sulcus, and no typical tubiform trail. Kutorginella aprica Grant, Reference Grant1976 (pl. 38, figs. 1–18) differs from the present species by its very shallow sulcus and more ventral strut spines.
Family Echinoconchidae Stehli, Reference Stehli1954
Genus Karavankina Ramovš, Reference Ramovš1969
Type species
Karavankina typica Ramovš, Reference Ramovš1969 from the Kazanian of northern Yugoslavia.
Remarks
Karavankina shares nearly identical spinosity with Calliprotonia Muir-Wood and Cooper, Reference Muir-Wood and Cooper1960, which has more than three rows of spines, with smaller spines anterior to larger spines on the concentric lamellose bands. Karavankina has much wider spine-free regions between the concentric bands of the ventral valves, more concave dorsal valves, and much weaker dorsal cardinal ridges. Waagenoconcha Cooper and Grant, Reference Cooper and Grant1975 mainly differs from Karavankina by its very dense, quincunxially arranged, pustulose, less prominent concentric bands, whereas Juresania Fredericks, 1928 differs by its much weaker posteriorly lamellose bands and quincunically arranged prostrate spines over the entire valves. Vediproductus is like Karavankina in its distinct concentric lamellose bands with numerous spines, but differs in its larger size, the absence of a spine-free zone, and prostrate spines covering the entire lamellose area (Shen et al., Reference Shen, Sun, Zhang and Yuan2016).
Karavankina typica Ramovš, Reference Ramovš1969
Figure 8.15–8.23
- Reference Ramovš1969
Karavankina typica Ramovš, p. 262, pl. 1, figs. 1–4.
- Reference Waterhouse1981
Karavankina cf. Karavankina typica; Waterhouse, p. 72, pl. 7, figs. 3–5, pl. 8, fig. 1.
Holotype
UL 3714/160, Kazanian, northern Yugoslavia (Ramovš, Reference Ramovš1969, pl. 1, fig. 1a–c).
Occurrence
Units 1 and 4–8, Taungnyo Group, Zwekabin Range, Myanmar; Ko Yao Noi Formation, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand; Roadian, northern Yugoslavia.
Description
Dorsal valve 10.8–20 mm long, 15–24.7 mm wide, somewhat subcircular in outline, widest approximately at midvalve; hinge line relatively straight, moderately concave, sometimes geniculated, forming short trail; cardinal extremities at 100–120° angle; very low, narrow median fold preserved on a few specimens; concentric lamellose bands very well developed, ~8–10 per 5 mm posteriorly, 5–7 per 5 mm anteriorly, each bearing 3–5 rows of spines; posterior one or two rows of spines of larger diameter; two or three rows of smaller spines anterior to larger spines; spine-free region spaced at upper one-third to one-half of each band. Dorsal interior with median septum reaching near midvalve.
Materials
Registered specimens: five external molds of dorsal valve and one internal mold of dorsal valve (NIGP 173973–173978).
Remarks
The present specimens are evidently allied to Karavankina owing to their small size, moderately concave dorsal valve, developed concentric bands, and more than three rows of spines arranged in bands over the entire dorsal valve. Few species of this genus can be favorably compared with our specimens except for the type species. Karavankina fascilata (Kutorga, Reference Kutorga1844) is relatively similar, but has much denser concentric lamellose bands than the present species. Other species vary greatly in both outline and size. However, Karavankina typica is closer to some species of Calliprotonia only based on these dorsal valves. It most closely resembles Calliprotonia inexpectum Cooper, Reference Cooper1957 (p. 48, pl. 8, figs. 13–26), but differs slightly in the stronger concavity of the dorsal valve and concentric bands with approximately one-third to one-half spine-free zone, whereas the bands of the latter form are almost fully occupied by several rows of spines. Calliprotonia renfraum Muir-Wood and Cooper, Reference Muir-Wood and Cooper1960 (p. 247, pl. 81, figs. 1–13) mainly differs from the present species by its larger size, dorsal valve with a much more elongate outline and weaker concavity, and denser spines.
Genus Vediproductus Sarycheva in Ruzhentsev and Sarycheva, Reference Ruzhentsev and Sarycheva1965
Type species
Vediproductus vediensis Sarycheva in Ruzhentsev and Sarycheva, Reference Ruzhentsev and Sarycheva1965 from the Wordian of Transcaucasia.
Remarks
The distinctions between Vediproductus and other homogenous genera of Echinoconchidae have been clearly clarified by Shen et al. (Reference Shen, Sun, Zhang and Yuan2016). The juvenile specimens of Vediproductus are particularly easily confused with Juresania in prostrate spine patterns (Shen et al., Reference Shen, Sun, Zhang and Yuan2016). However, the concentric bands of mature Vediproductus always carry three different kinds of spines whereas Juresania features a single type of prostrate spines.
Vediproductus dissimilis (Waterhouse, Reference Waterhouse1981)
Figure 9.1–9.5
- Reference Waterhouse1981
Juresania dissimilis Waterhouse, p. 71, pl. 4, figs. 9, 10, pl. 5, figs. 1–3, pl. 6, figs. 1–3, pl. 7, figs. 1, 2.

Figure 9. (1–5) Vediproductus dissimilis (Waterhouse, Reference Waterhouse1981): (1–3) internal mold, incomplete external mold of dorsal valve and detail of its spinosity, showing three kinds of spines (circles), NIGP 173979; (4) incomplete external mold of dorsal valve, NIGP 173980; (5) external mold of dorsal valve, NIGP 173981. (6) Magniplicatina sp. indet., external mold of dorsal valve, NIGP 173985. (7) Cimmeriella mucronata (Fang, Reference Fang1994), incomplete internal mold of ventral valve, NIGP 173986. (8, 9) Derbyia perplexus (Waterhouse, Reference Waterhouse1981), external molds of ventral valve, NIGP 173997, 173998. (10–21) Stenoscisma quasimutabilis Waterhouse, Reference Waterhouse1981: (10, 11) internal molds of conjoined shell, NIGP 173999, 174000; (12–15) posterior and ventral views of internal mold of conjoined shell and their latex casts of ventral and dorsal interior, NIGP 174001; (16) latex cast of dorsal valve, NIGP 174002; (17) internal mold of dorsal valve, NIGP 174003; (18, 19) internal molds of ventral valve, NIGP 174004, 174005; (20, 21) internal mold of ventral valve and its latex cast, NIGP 174006. Scale bars = 5 mm, unless otherwise labeled.
Holotype
TBR 33, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand (Waterhouse, Reference Waterhouse1981, pl. 5, fig. 1).
Occurrence
Units 3, 6, and 8, Taungnyo Group, Zwekabin Range, Myanmar; Ko Yao Noi Formation, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand.
Description
Dorsal valve 25.5–34.7 mm long, 39.2–47.7 mm wide, flattened to gently concave, not geniculated, transversely subrectangular in outline; greatest width approximately at midvalve, slightly wider than hinge; dorsal cardinal extremities rounded; ears slightly convex, very small, inconspicuous; median fold very low, weak, originating from midvalve, approximately at one-third of valve width anteriorly; growth lamellae obvious, five or six per 10 mm; spines close-set on concentric bands; spine bases 0.1–0.8 mm in diameter, usually in three types: larger spines slightly prostrate, smaller ones erect, and many hair-like spinules. Dorsal cardinal process bilobate; two short buttress plates converging in front of cardinal process; cardinal ridges long, extending along hinge; anterior adductor scars small, tear-drop shaped; posterior adductors dendritic; median septum extending approximately to anterior third of valve length; endospines numerous in concentric rows.
Materials
Registered specimens: one internal mold of dorsal valve and three external molds of dorsal valve (NIGP 173979–173981).
Remarks
The present specimens were very difficult to assign to either Vediproductus or Juresania because only dorsal valves are available. From the observations of the dorsal cardinal area, these specimens are probably better included in Juresania because the cardinal process is supported by two short buttress plates separated by the median septum, not directly connected to the median septum as in Vediproductus. However, the concentric bands of our specimens are greatly developed, whereas those in the dorsal interior of Juresania are very weak or absent. More importantly, three different types of spines are clearly exhibited on the lamellose bands (Fig. 9.3), which is the distinctive feature of Vediproductus. Overall, these specimens from eastern Myanmar are probably the transitional species between Juresania and Vediproductus. Considering that the strength of the concentric lamellose bands and spine patterns are the main basis for distinguishing these two genera, the present specimens are assigned to Vediproductus. Similarly, the southern Thailand forms once named as Juresania dissimilis are better revised to Vediproductus dissimilis (Waterhouse, Reference Waterhouse1981) according to the large shell size (dorsal valve to 60 mm wide) and obvious concentric lamellose bands over both valves. Dorsal features of the present specimens fit well within this species except for some interspecific differences, e.g., fewer concentric lamellae and larger spines. Our specimens are very similar to Vediproductus punctatiformis in outline, size of the spines, and the concentric lamellose pattern, but differ by their flat dorsal disk without geniculation, the presence of buttress plates, and more erect spines.
Family Linoproductidae Stehli, Reference Stehli1954
Genus Cimmeriella Archbold and Hogeboom, Reference Archbold and Hogeboom2000
Type species
Productus tenuistriatus var. foordi Etheridge, Reference Etheridge1903 from the late Sakmarian of Western Australia.
Remarks
Cimmeriella is easily confused with Globiella Muir-Wood and Cooper, Reference Muir-Wood and Cooper1960 and Stepanoviella Zavodowsky, Reference Zavodowskiy and Markowskii1960 in view of their similar external appearance, e.g., small to medium size, globose outline, small ears, and dominant costellate ornament. Globiella is characterized by deeply impressed and markedly striate diductor scars and obscurely lobate adductors, whereas in Cimmeriella, diductors are only feebly striate and the adductor scars are deeply impressed with a dendritic pattern (Archbold and Hogeboom, Reference Archbold and Hogeboom2000). Stepanoviella species are adorned with extremely fine costellae and are generally restricted to the Kazanian-Tatarian of northeastern Russia (Grigor'ewa et al., Reference Grigor'eva, Ganelin, Kotlyar and Sarycheva1977). In contrast, the present genus features relatively coarse costellae and wide interspaces. Most species of this genus are found from the Permian Cimmerian marine province, from which the name of this genus originates.
Cimmeriella mucronata (Fang, Reference Fang1994)
Figures 9.7, 10.1, 10.2
- Reference Jin and Sun1981
Stepanoviella flexuosa Jin and Sun, p. 140, pl. 5, figs. 7, 8.
- Reference Nie, Song, Jiang and Liang1993
Stepanoviella hemisphaerium Nie et al., pl. 1, figs. 6–8.
- Reference Fang1994
Stepanoviella flexuosa; Fang, p. 267, pl. 1, figs. 6–9.
- Reference Fang1994
Stepanoviella mucronata Fang, p. 268, pl. 1, figs. 10–13.
- Reference Shi and Waterhouse1996
Globiella youwangensis Shi et al., p. 92, fig. 4F.
- Reference Shen, Shi and Zhu2000
Cimmeriella mucronata; Shen et al., p. 272, pl. 2, figs. 10–15.
- Reference Zhan, Yao, Ji and Wu2007
Cimmeriella mucronata; Zhan in Zhan et al., pl. 3, figs. 12–14, 17.

Figure 10. (1, 2) Cimmeriella mucronata (Fang, Reference Fang1994), internal molds of ventral valve, NIGP 173982, 173983. (3) Kasetia kaseti Waterhouse, Reference Waterhouse1981, internal mold of ventral valve, NIGP 173984. (4) Cleiothyridina sp. indet., external mold of ventral valve, NIGP 174007. (5–12) Spinomartinia prolifica Waterhouse, Reference Waterhouse1981: (5–11) internal molds of ventral valve, NIGP 174008–174014; (12) internal mold of dorsal valve, NIGP 174015. (13, 14) Martinia sp. indet., internal molds of conjoined shell, NIGP 174016, 174017. (15–17) Neospirifer sp. indet., ventral and dorsal views of compressed internal mold of conjoined shell and external mold of dorsal valve, NIGP 174018. (18–24) Spiriferella modesta Waterhouse, Reference Waterhouse1981: (18) internal mold of conjoined shell, NIGP 174019; (19, 20) internal mold of ventral valve and its latex cast, NIGP 174020; (21–24) internal molds of ventral valve, NIGP 174021–174024. Scale bars = 5 mm.
Holotype
YNGS 91303, Dingjiazhai Formation, Baoshan, western Yunnan, China (Fang, Reference Fang1994, pl. 1, fig. 10)
Occurrence
Units 2, 5, and 7, Taungnyo Group, Zwekabin Range, Myanmar; Dingjiazhai Formation, Baoshan, western Yunnan, China; Wululong Formation, Linzhou, China; upper part of the Yunzhug Formation, Xainza, Tibet, China.
Materials
Registered specimens: three internal molds of ventral valve (NIGP 173982, 173983, 173986).
Remarks
The external features of these specimens fit well with Cimmeriella including their globular appearance, no sulcus, small ears, and costellate ornament. They are distinguished from Stepanoviella by much coarser costellae with wide intercostal valleys, ~7 per 5 mm at midvalve, and from Globiella by obviously dendritic ventral adductor scars. The present specimens probably belong to Cimmeriella mucronata in view of their small size, transverse outline, and nearly identical costellate pattern.
Genus Kasetia Waterhouse, Reference Waterhouse1981
Type species
Kasetia kaseti Waterhouse, Reference Waterhouse1981 from the early Cisuralian of southern Thailand.
Remarks
The present genus is very similar to Cancrinella Fredericks, Reference Fredericks1928 and Costatumulus Waterhouse, Reference Waterhouse1983a in general appearance. It differs from Cancrinella in its more convex ventral disc, much denser and stronger rugae, no dorsal spines, and few dorsal dimples (Waterhouse Reference Waterhouse1981); it differs from Costatumulus in its somewhat irregular and discontinuous rugae and dendritic (not striate) adductor scars.
Kasetia kaseti Waterhouse, Reference Waterhouse1981
Figure 10.3
- Reference Waterhouse1981
Kasetia kaseti Waterhouse, p. 90, pl. 18, figs. 6–15, pl. 19, figs. 1, 2.
- Reference Shi and Waterhouse1991
Kasetia kaseti; Shi and Waterhouse, Reference Shi and Waterhouse1991, p. 34, fig. 3.1.
- Reference Shi, Leman Mohd, Tan, Dheeradilok, Hinthong, Chadomrong, Putthapiban, Tansathien, Utha-aroon, Sattyarak, Nuchanong and Techawan1997
Kasetia cf. Kasetia kaseti; Shi et al., figs. 3A, 4C.
- Reference Shi, Raksaskulwong, Campbell, Hill, Henderson and Bamber2002
Kasetia kaseti; Shi et al., pl. 1, fig. 4.
Holotype
TBR 287, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand (Waterhouse, Reference Waterhouse1981, pl. 18, figs. 12, 14).
Occurrence
Unit 6, Taungnyo Group, Zwekabin Range, Myanmar; Ko Yao Noi Formation, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand; upper Khao Phra Formation, Khao Phra, southern Thailand; Singa Formation, Langkawi Island, northwestern peninsular Malaysia.
Description
Ventral valve 11.4 mm long, 19.1 mm wide, moderately convex in profile, semicircular to subrectangular in outline; greatest width slightly anterior to hinge; cardinal extremities rounded; beak low, broad, with ~120° angle, slightly over hinge line; umbonal region swollen; eras small, flattened, well demarcated from disc; sulcus absent; roundly-crested costellae fine, dense, bifurcating anteriorly, ~12–15 per 5 mm at anterior margin, with interspaces wider than costellae; concentric rugae irregular, slightly discontinuous, becoming sparser and much stronger laterally, slightly converging to umbonal region, ~18 over entire valve; spines erect, developed along hinge in two or three rows, mostly at hinge, very rare to almost absent over visceral disc and trail.
Materials
Registered specimens: one internal mold of ventral valve (NIGP 173984).
Remarks
Kasetia kaseti, the only known species of the genus Kasetia, is easily confused with Costatumulus sp. indet., once identified as Cancrinella cancriniformis (Tschernyschew, Reference Tschernyschew1902), from the Ko Yao Noi Formation of southern Thailand (Waterhouse, Reference Waterhouse1981, p. 86, pl. 17, figs. 10–12, pl. 18, figs. 1–5), but is mainly distinguished by its fewer ventral spines without prolonged bases and no dorsal spines. The present specimen is almost identical with those from the Singa Formation of Malaysia and the upper Khao Phra Formation of Thailand (Shi et al., Reference Shi, Leman Mohd, Tan, Dheeradilok, Hinthong, Chadomrong, Putthapiban, Tansathien, Utha-aroon, Sattyarak, Nuchanong and Techawan1997, Reference Shi, Raksaskulwong, Campbell, Hill, Henderson and Bamber2002), and compares well with the holotype except for its somewhat more transverse outline.
Family Monticuliferidae Muir-Wood and Cooper, Reference Muir-Wood and Cooper1960
Genus Magniplicatina Waterhouse, Reference Waterhouse1983b
Type species
Cancrinella magniplica Campbell, Reference Campbell1953 from the Kungurian of the Bowen Basin, eastern Australia.
Magniplicatina sp. indet.
Figure 9.6
Occurrence
Unit 1, Taungnyo Group, Zwekabin Range, Myanmar.
Materials
Registered specimens: one external mold of dorsal valve (NIGP 173985).
Remarks
This specimen is probably referable to Magniplicatina in view of its general outline and ornamentation especially the irregular, very strong growth rugae. In addition, this specimen is relatively large, > 43 mm long and 42 mm wide, and thus cannot be assigned to other similar linoproductid, e.g., Costatumulus. Further identification at the species level is hampered by the poor preservation of the present specimen.
Family Aulostegidae Muir-Wood and Cooper, Reference Muir-Wood and Cooper1960
Genus Urushtenia Licharew, Reference Licharew1935
Type species
Productus pseudomedusa Tshernyschew, Reference Tschernyschew1902 from the Cisuralian of Russia.
Remarks
Urushtenoidea Jin and Hu, Reference Jin and Hu1978, the closest relative of the present genus, has been studied by Jin (Reference Jin1963), Jin and Hu (Reference Jin and Hu1978), Nakamura (Reference Nakamura1979), and Zeng (Reference Zeng1987) in detail. However, the differences between these two genera have not been clarified, especially regarding internal structures. The presence of a dorsal median septum and the absence of endospines or marginal fence spines in Urushtenia were proposed by Jin and Hu (Reference Jin and Hu1978) as the distinguishing features from Urushtenoidea. Actually, after careful comparison of specimens of their type species, there are almost no differences in the dorsal internal structures of these two genera (Sarycheva and Grunt, Reference Sarycheva and Grunt1969, text-fig. 3; Zeng, Reference Zeng1987, text-fig. 4). Shared features are a robust bifid cardinal process, a distinct alveolus, a long median septum, a tear-drop shaped brachial ridge, and marginal fence spines. The external appearance of the ventral trail in Urushtenoidea most obviously differs in having scaly concentric lamellae with complex spinosity (two kinds of spines in opposite directions forming a bidirectional serration, one in the costae and the other in the interspace; vertical spinules also occur in the interspaces), whereas that of Urushtenia is normally ornamented with robust projected marginal spines and a smooth, thickened marginal stereozone. Additionally, Urushtenia has a weaker geniculation on the ventral valve but a strongly geniculated dorsal valve with a longer trail than in Urushtenoidea. It is noteworthy that the stratigraphic distributions of Urushtenia and Urushtenoidea vary significantly; the former is generally limited to the Cisuralian, whereas the latter is normally late Guadalupian.
Urushtenia arguta Grant, Reference Grant1976
Figure 8.4–8.14
- Reference Grant1976
Urushtenia arguta Grant, p. 93, pl. 20, figs. 23–40.
- Reference Waterhouse1981
Urushtenia sp. indet., Waterhouse, p. 68, pl. 4, fig. 3.
Holotype
USNM 212075, Ratiburi Group, Ko Muk, southern Thailand (Grant, Reference Grant1976, pl. 20, figs. 36–40).
Occurrence
Units 1–7, Taungnyo Group, Zwekabin Range, Myanmar; Ko Yao Noi Formation, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand; Ratiburi Group, Ko Muk, southern Thailand.
Description
Shell 11.3–12.9 mm long, 17.3–19.4 mm wide, transversely subquadrate in outline, widest at hinge; cardinal extremities slightly obtuse. Ventral valve moderately convex; beak short, broad; ears distinctly demarcated from visceral region; sulcus very weak, probably originating from midvalve; costae low with sharp crest, approximately five per 5 mm anteriorly; interspace becoming a round pit at anterior margin; several concentric rugae observed laterally from internal molds; strong rhizoid spine emanating from terminated point of costae, ventrally directed; smooth, thickened marginal stereozone at front of costae. Dorsal valve slightly concave to flat, strongly geniculated to form trail anteriorly; trail approximately at one-third of valve length; ears small, gently convex; fold inconspicuous over anterior half, relatively low; fine, regular reticulate ornament covering entire visceral region; costae slightly coarse, narrower than intertroughs, approximately five or six per 5 mm anteriorly; concentric rugae becoming slightly sparser and fainter anteriorly, ~10 per 5 mm along hinge; many elongated pits over dorsal valve; spines scattered on disc, arranged in row at anterior end of valve.
Ventral interior with clear muscle markings; pair of adductor scars posteriorly elongate, narrow; diductor scars anteriorly broad. Dorsal interior with bifid cardinal process; adductor scars a large alveolus anteriorly supported by prominent median septum, reaching midvalve, slightly narrowing forward; lateral ridges relatively weak, only marked near cardinal process; brachial ridges distinct, enclosing tear-drop shaped region on valve floor; disc surrounded by thick marginal ridge; endospines or marginal fence spines arranged in row at trail.
Materials
Registered specimens: three internal molds of ventral valve, one dorsal interior, two internal molds of dorsal valve, two external molds of dorsal valve, and two external molds of ventral trail and their latex casts (NIGP 173987–173996).
Remarks
The present specimens are credibly assigned to Urushtenia rather than Urushtenoidea in view of the absence of concentric lamellae with complex spinosity at the ventral trail, the presence of a thickened marginal stereozone, and a strongly geniculated dorsal valve with a relatively long trail. The single ventral internal mold, identified as Urushtenia sp. indet. by Waterhouse (Reference Waterhouse1981, p. 68, pl. 4, fig. 3), is assigned to the present species based on almost equal features. This species mainly differs from Urushtenia murina Grant, Reference Grant1976 by its slightly larger size, much more transverse outline, and maximum width at the hinge. The type species, Urushtenia pseudomedusa (Tschernyschew, Reference Tschernyschew1902), is close to the present species in outline, but is distinguished by its much coarser, sparser costae as well as its longer dorsal spines.
Order Orthotetida Waagen, Reference Waagen1884
Family Derbyiidae Stehli, Reference Stehli1954
Genus Derbyia Waagen, Reference Waagen1884
Type species
Derbyia regularis Waagen, Reference Waagen1884 from the Guadalupian of the Salt Range, Pakistan.
Derbyia perplexus (Waterhouse, Reference Waterhouse1981)
Figure 9.8, 9.9
- Reference Waterhouse1981
Orthotetes perplexus Waterhouse, p. 58, pl. 1, figs. 1–7.
Holotype
TBR 3, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand (Waterhouse, Reference Waterhouse1981, pl. 1, fig. 1).
Occurrence
Units 1 and 6, Taungnyo Group, Zwekabin Range, Myanmar; Ko Yao Noi Formation, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand.
Description
Ventral valve small to medium (for genus), 18.9–28.6 mm long, 25.9–38.4 mm wide, semicircular in outline, slightly convex, widest at hinge; cardinal extremities obtuse, rounded; fold relatively low, ~10 mm wide near anterior margin; costellae extremely dense, increasing by intercalation approximately at anterior quarter, ~20 per 10 mm anteriorly, crossed by concentric wrinkles; wrinkles almost evenly spaced, 2 mm apart.
Materials
Registered specimens: two external molds of ventral valve (NIGP 173997, 173998).
Remarks
The external molds of ventral valves are almost identical with those assigned to Orthotetes perplexus from the Ko Yao Noi Formation of southern Thailand (Waterhouse, Reference Waterhouse1981). According to the description by Waterhouse (Reference Waterhouse1981), the cardinal process on the dorsal valve is supported by divergent, but not recurved, crural plates with a low socket ridge; a small spondylium is not observed, which suggests that those specimens belong to Derbyia rather than Orthotetes Fischer de Waldheim, Reference Fischer de Waldheim1829 (Williams et al. Reference Williams, Brunton, Wright and Kaesler2000).
Order Rhynchonellida Kuhn, Reference Kuhn1949
Family Stenoscismatidae Oehlert, Reference Oehlert and Fischer1887
Genus Stenoscisma Conrad, Reference Conrad1839
Type species
Terebratula schlottheimi von Buch, Reference Buch1835 from the Lopingian of Germany.
Stenoscisma quasimutabilis Waterhouse, Reference Waterhouse1981
Figure 9.10–9.21
- Reference Waterhouse1981
Stenoscisma quasimutabilis Waterhouse, p. 91, pl. 19, figs. 3–17, pl. 20, figs. 1–6.
- Reference Shi and Waterhouse1991
Stenoscisma cf. Stenoscisma quasimutabilis; Shi and Waterhouse, p. 34, fig. 3.5–3.7.
- Reference Shi, Raksaskulwong, Campbell, Hill, Henderson and Bamber2002
Stenoscisma quasimutabilis; Shi et al., pl. 2, fig. 15.
Holotype
TBR 150, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand (Waterhouse, Reference Waterhouse1981, pl. 19, fig. 17).
Occurrence
Units 1–6 and 8, Taungnyo Group, Zwekabin Range, Myanmar; Ko Yao Noi Formation, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand; upper Khao Phra Formation, Khao Than, southern Thailand.
Description
Shell medium, 12.3–20.5 mm long, 14.9–27 mm wide, with maximum width approximately at anterior third of shell length, subtrigonal to subpentagonal in outline, biconvex in profile; cardinal extremities at obtuse angle. Ventral valve moderately convex; beak extended, slightly incurved, of ~100° angle; sulcus very wide, extremely shallow, commencing anterior to umbonal region; costae beginning from posterior third of valve length, low; crests blunt, approximately three or four per 5 mm, occasionally intercalating approximately at midvalve. Dorsal valve less convex; fold almost absent in immature shells; four costae distinct on central part, weakening laterally; two growth wrinkles present approximately at midvalve. Stolidium not observed.
Ventral interior with small teeth; spondylium sessile in beak posteriorly, supported by long median septum reaching approximately posterior third of valve length; muscle scars not clear within spondylium, probably dendritic. Dorsal interior with small camarophorium supported by high median septum; hinge plate and intercamarophorial plate converging at posterior part of camarophorium; single pair of very short crura passing forward from anterior edge of hinge plate.
Materials
Registered specimens: three internal molds of conjoined shell, three internal molds of ventral valve, one latex cast of dorsal valve and one internal mold of dorsal valve (NIGP 173999–174006).
Remarks
The described specimens are confidently assigned to Stenoscisma based on their subtrigonal outline, distinct costae on both valves, and especially the internal structures (e.g., spondylium, camarophorium, and median septum) although a stolidium was not observed. Stenoscisma quasimutabilis was erected by Waterhouse (Reference Waterhouse1981) based on Thailand specimens that are very similar to Stenoscisma mutabilis (Tschernyschew, Reference Tschernyschew1902). But the costae of the present species arise later, and are spaced denser but much lower and less prominent when compared with Stenoscisma mutabilis. Another Thailand form, Stenoscisma tetricum Grant, Reference Grant1976, is distinguished from the present species by its more elongate outline, its costae beginning from beak, and its prominent stolidium.
Order Athyridida Boucot, Johnson, and Staton, Reference Boucot, Johnson and Staton1964
Family Athyrididae Davidson, Reference Davidson1881
Genus Cleiothyridina Buckman, Reference Buckman1906
Type species
Atrypa pectinifera Sowerby, 1840 in Reference Sowerby1840–1846 from the Lopingian of the UK.
Remarks
This genus can be distinguished from other genera of athyrids based on internal structures, e.g., dental plates in the ventral interior, an apically perforate dorsal hinge plate, and external ornamentation (e.g., closely spaced concentric lamellae fringed by flat spines) (Grant, Reference Grant1976).
Cleiothyridina sp. indet.
Figure 10.4
Occurrence
Unit 4, Taungnyo Group, Zwekabin Range, Myanmar.
Description
Ventral valve 19.8 mm long, 26.1 mm wide, slightly convex, transversely oval in outline; maximum width approximately at midvalve; umbonal region distorted; beak unclear; sulcus inconspicuous; concentric lamellae relatively dense but irregular, approximately one or two per mm, bearing flattened, pectinate spines, ~12 per 5 mm anteriorly; fringe-like spines distinct at anterior margin, ~8 mm long.
Materials
Registered specimens: one external mold of ventral valve (NIGP 174007).
Remarks
The present specimen can be safely assigned to Cleiothyridina owing to its typical pectinate, fringed spines. It is similar to Cleiothyridina seriata Grant, Reference Grant1976 in outline and size, but mainly differs in its sparser growth lamellae and much longer fringe-like spines. The features of this specimen are almost consistent with those of Cleiothyridina sp. indet. from the Ko Yao Noi Formation of southern Thailand (Waterhouse Reference Waterhouse1981). The present specimen probably belongs to Cleiothyridina pectinifera Sowerby, 1840 in Reference Sowerby1840–1846 in view of the external features of the ventral valve, but unfortunately the internal structures of the ventral valve as well as the dorsal valve are not available for comparison.
Order Spiriferida Waagen, Reference Waagen1883
Family Martiniidae Waagen, Reference Waagen1883
Genus Spinomartinia Waterhouse, Reference Waterhouse1968
Type species
Spinomartinia spinosa Waterhouse, Reference Waterhouse1968 from the Wuchiapingian of New Zealand.
Remarks
Spinomartinia was established to differ from Martinia M'Coy, Reference M'Coy1844 based on micro-ornament consisting of fine erect spines mainly on the dorsal valve. However, the preservation of such spinules is rare, making the genus difficult to recognize (Waterhouse, Reference Waterhouse1968). On this basis, Shi and Waterhouse (Reference Shi, Fang and Archbold1996) suggested that internal structures in the ventral interior might be more applicable taxonomically, i.e., Spinomartinia is characterized by strong, ramiform to netlike pallial markings, whereas Martinia is distinguished by pinnate pallial markings. However, recently, He et al. (Reference He, Shi, Zhang, Yang, Shen and Zhang2019) proved that the distinctive ramiform to netlike pallial markings are also found in the ventral interior of some species of Martinia in South China, e.g., Martinia liuqiaoiensis He, Shen, and Shi in He et al., Reference He, Shi, Zhang, Yang, Shen and Zhang2019 and Martinia ziyunensis Chen in Chen et al., Reference Chen, Shi, Gao, Tong, Yang and Peng2009. Thus, the feature of pallial markings within the ventral valve possibly cannot be regarded as a key difference between these two genera (He et al., Reference He, Shi, Zhang, Yang, Shen and Zhang2019). In addition, combing the characters of all determined species of Spinomartinia, it is obvious that they all feature fine spinules on the dorsal valve, ramiform to netlike pallial markings in the ventral interior, and an inconspicuous fold and sulcus. By contrast, most of the species of Martinia are characterized by pinnate pallial markings and a prominent fold and sulcus. Considering that the fine spinules as a diagnostic feature are hardly observed except in well-preserved external molds of the dorsal valve, the specimens with ramiform to netlike pallial markings and an inconspicuous fold and sulcus in South China can be also included in Spinomartinia; this possibility cannot be ruled out.
Spinomartinia prolifica Waterhouse, Reference Waterhouse1981
Figure 10.5–10.12
- Reference Waterhouse1981
Spinomartinia prolifica Waterhouse, p. 107, pl. 27, figs. 10–12, pl. 28, figs. 1–15, pl. 29, figs. 1–12, pl. 31, fig. 5.
- Reference Shi and Waterhouse1991
Spinomartinia prolifica; Shi and Waterhouse, p. 37, fig. 5.18–5.25.
- Reference Shi, Raksaskulwong, Campbell, Hill, Henderson and Bamber2002
Spinomartinia prolifica; Shi et al., pl. 2, figs. 10, 13.
- Reference Win2009
Spinomartinia cf. Spinomartinia prolifica; Zaw Win, fig. 2.3.
Holotype
TBR 204, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand (Waterhouse, Reference Waterhouse1981, pl. 28, fig. 6, pl. 29, fig. 6).
Occurrence
Units 1–8, Taungnyo Group, Zwekabin Range, Myanmar; Ko Yao Noi Formation, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand; upper Khao Phra Formation, Khao Than, southern Thailand; Nam Loong beds, Kinta Valley, western Malaysia.
Description
Shell small, maximum length ranging 11–20 mm, maximum width ranging 12.5–37 mm, transversely oval in outline, biconvex in profile; ventral valve more convex than dorsal valve; greatest width placed between shell midlength and anterior third; cardinal extremities well rounded, with ~110–120° angle. Ventral valve moderately convex; beak pointed, incurved, distinctly extending beyond hinge line; hinge narrow with interarea strongly concave; short sulcus very wide, shallow anteriorly in minority of specimens; external ornament not observed; lanceolate muscle field deeply impressed on posterior region on median groove; radial pallial grooves very common on valve floor, distinct, normally ramiform. Dorsal valve gently convex; interior with median groove and very fine pallial grooves.
Materials
Registered specimens: seven internal molds of ventral valve and one internal mold of dorsal valve (NIGP 174008–174015).
Remarks
These specimens are almost the same as those of Spinomartinia prolifica in southern Thailand (Waterhouse, Reference Waterhouse1981) in view of their size, outline, obvious ramiform vascular impressions in ventral internal molds, and stratigraphic occurrence, although no external molds of a dorsal valve are available to check for fine spinules. This species can be readily distinguished from Spinomartinia spinosa from the Wuchiapingian of New Zealand by its oval outline. Spinomartinia queenslandica Waterhouse, Reference Waterhouse1987 from the Artinskian of eastern Australia is close in general appearance, but the dorsal valve is more convex and the ventral sulcus is almost absent.
Genus Martinia M'Coy, Reference M'Coy1844
Type species
Spirifer glaber Sowerby, 1820 in Reference Sowerby1818–1821 from the early Carboniferous of the UK.
Martinia sp. indet.
Figure 10.13, 10.14
Occurrence
Units 6 and 8, Taungnyo Group, Zwekabin Range, Myanmar.
Materials
Registered specimens: two internal molds of conjoined shell (NIGP 174016, 174017).
Remarks
These two specimens are readily distinguished from those discovered of Spinomartinia prolifica in view of their much more convex shell, much fewer and finer radial pallial grooves in the ventral interior and absence of a deeply impressed lanceolate muscle field. Unfortunately, external molds of the dorsal valve are not preserved, thus it is impossible to know whether the dorsal exterior has fine spinules. We suggest that these specimens be assigned to Martinia rather than Spinomartinia because this species is so different from any known species of Spinomartinia based on its strong shell convexity.
Family Trigonotretidae Schuchert, Reference Schuchert1893
Genus Neospirifer Fredericks, Reference Fredericks1924
Type species
Spirifer fasciger Keyserling, Reference Keyserling1846 from the Cisuralian of Timan Peninsula, Arctic.
Neospirifer sp. indet.
Figure 10.15–10.17
Occurrence
Unit 4, Taungnyo Group, Zwekabin Range, Myanmar.
Description
Shell > 41.4 mm long, > 49.2 mm wide, transverse in outline, biconvex in lateral profile, but convexity of both valves is unknown owing to compressed preservation; greatest width probably at hinge according to general outline; cardinal extremities not preserved. Ventral beak moderately incurved; interarea high, slightly concave; sulcus originating from umbo, relatively narrow with ~20° angle; five pairs of plicae spaced on each flank; costae bifurcating near beak and again anterior to umbonal region, forming six fasciles anteriorly over each plica; numerous (> 10) costae densely distributed in sulcus; only two distinct concentric wrinkles observed on ventral valve. Dorsal beak obtuse, angle > 140°; fold high, narrow, with ridge-shaped crest; fasciculate costae distinct over five pairs of plicae on each flank, 6–10 near anterior margin, 8–10 bifurcated from fold on both sides; growth lamellae well-preserved on external mold of dorsal valve, regularly spaced, approximately two per mm.
Ventral interior with teeth supported by dental plates and adminicula; muscle field elongate-oval, divided by median ridge; diductors linear. Dorsal interior with obvious striated cardinal process.
Materials
Registered specimens: one incomplete compressed internal mold of conjoined shell and one external mold of dorsal valve (NIGP 174018).
Remarks
The present specimen can be assigned to Neospirifer based on its obvious fasciculate costae. However, the features of sulcus, fold, and cardinal extremities cannot be totally restored because this specimen was compressed and incomplete that is hard to do further identification.
Family Spiriferellidae Waterhouse, Reference Waterhouse1968
Genus Spiriferella Tschernyshew, Reference Tschernyschew1902
Type species
Spirifer saranae de Verneuil, Reference Verneuil, Murchison, Verneuil and Keyserling1845 from the Cisuralian of Russia.
Spiriferella modesta Waterhouse, Reference Waterhouse1981
Figure 10.18–10.24
- Reference Waterhouse1981
Spiriferella modesta Waterhouse, p. 98, pl. 23, figs. 2–9, pl. 24, figs. 1–11, pl. 25, figs. 1, 2.
Holotype
TBR 174, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand (Waterhouse, Reference Waterhouse1981, pl. 24, figs. 1–7, pl. 25, fig. 1).
Occurrence
Units 1–7, Taungnyo Group, Zwekabin Range, Myanmar; Ko Yao Noi Formation, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand.
Description
Shell small to medium (for genus), greatest length > 22.5 mm, greatest width ranging 17.1–33.4 mm, thickness ~21.9 mm, widest anterior to hinge, biconvex in lateral profile; juvenile specimens elongate, adults transverse in outline; hinge narrow, concave; cardinal extremities subangular to rounded. Ventral valve moderately convex; interarea triangular, slightly incurved, with distinct triangular delthyrium; beak moderately incurved, projecting beyond hinge line; umbonal region greatly inflated; sulcus with ~20° angle, moderately deep, slightly widening anteriorly; plicae well developed on each flank, approximately five or six pairs, with rounded crests, gradually weakening laterally; microornament of fine radial capillae relatively clear on posterior region; Dorsal valve less convex; interarea low, planar, with poorly preserved notothyrium; umbonal region moderately swollen; fold very narrow, slightly raised without median groove; four or five pairs of plicae on each flank, interspaces much wider than plicae; one pair of subplicae on both sides of dorsal fold.
Ventral interior with teeth supported by slightly convergent dental plates; muscle field deeply impressed; adductor scars narrowly oval; diductor scars dendritic to slightly linear; posterior floor marked by numerous pits; median ridge distinct in front of muscle field.
Materials
Registered specimens: five internal molds of ventral valve and one internal mold of conjoined shell (NIGP 174019–174024).
Remarks
Spiriferella is characterized by its usually elongate, narrow outline, broad and rounded plicae with simple, wide costae laterally, deeply impressed muscle scars, and dental plates in the ventral interior. The features of the present specimens are consistent with those of Spiriferella except for the somewhat transverse outline of mature specimens. These specimens were identified with the holotype of Spiriferella modesta in southern Thailand. Waterhouse (Reference Waterhouse1981) made a detailed comparison of the present species with other species of Spiriferella that were erected before 1981. Spiriferella etheridgei Archbold and Thomas, Reference Archbold and Thomas1985 is like Spiriferella modesta in its smooth fold with no groove, but mainly differs by its much more elongate outline. To summarize, the present species is mainly distinguished from other species of Spiriferella by its much more transverse outline, smaller size, relatively deep sulcus, and low dorsal fold without a groove or slit.
Family Elythidae Fredericks, Reference Fredericks1924
Genus Phricodothyris George, Reference George1932
Type species
Phricodothyris lucerna George, Reference George1932 from the Lower Carboniferous of the UK.
Phricodothyris sp. indet.
Figure 11.1–11.3
Occurrence
Units 1, 3, and 5, Taungnyo Group, Zwekabin Range, Myanmar.
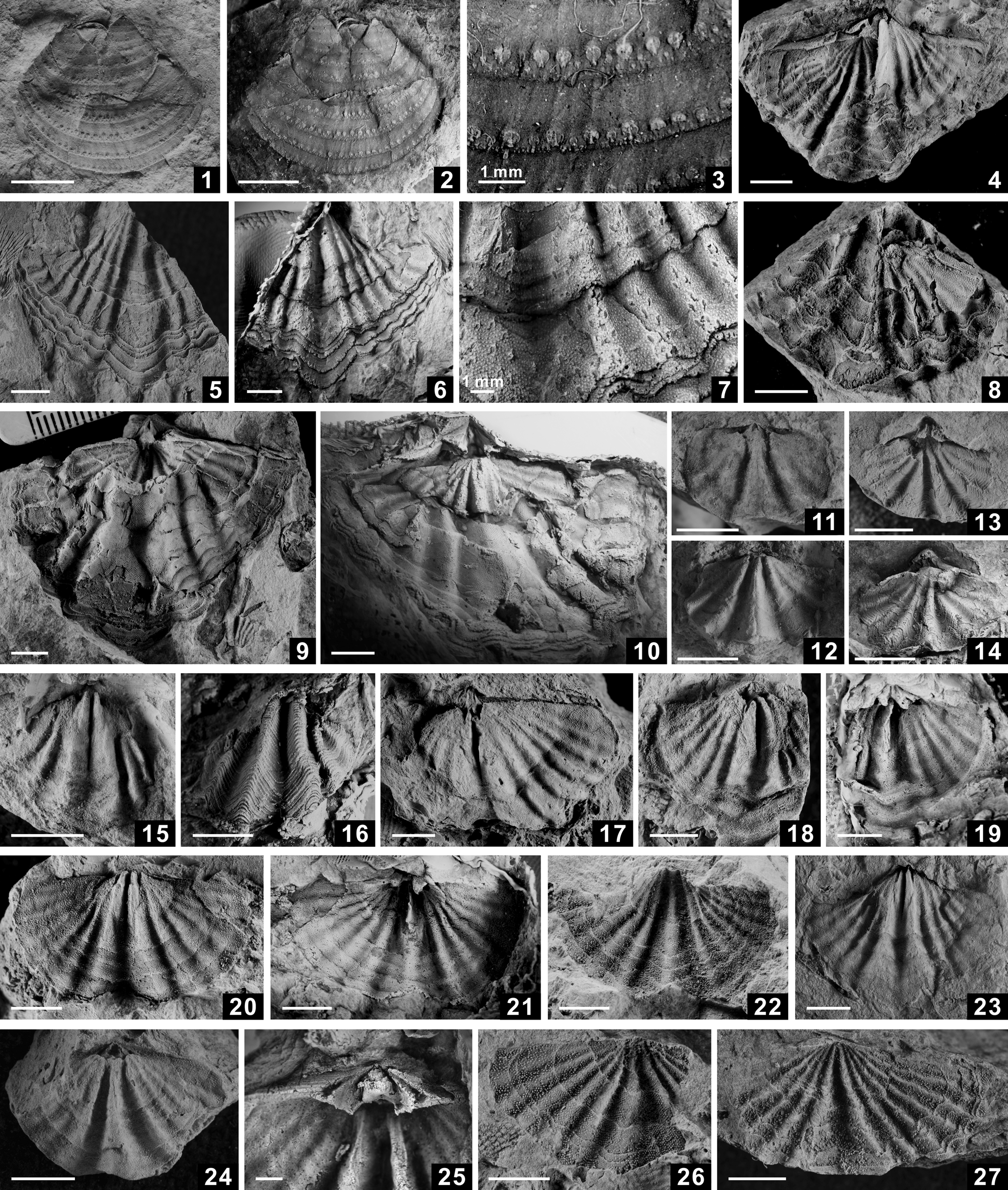
Figure 11. (1–3) Phricodothyris sp. indet., external mold of dorsal valve, its latex cast, and detail of spinosity, NIGP 174025. (4–10) Reticulariina cf. Reticulariina atava Chronic in Newell et al., Reference Newell, Chronic and Roberts1949: (4) external mold of dorsal valve partly covered by internal mold of ventral valve, NIGP 174026; (5–7) external mold of ventral valve, its latex cast, and detail of spinosity, NIGP 174027; (8) incomplete external mold of ventral valve, NIGP 174028; (9, 10) internal mold of ventral valve and external mold of dorsal valve of a small Reticulariina and their latex cast, NIGP 174029. (11–16) Yaonoiella mantajiti Waterhouse, Reference Waterhouse1983b: (11, 12) internal molds of dorsal valve, NIGP 174030, 174031; (13, 14) external mold of dorsal valve and its latex cast, NIGP 174032; (15) internal mold of ventral valve, NIGP 174033; (16) latex cast of ventral valve, NIGP 174034. (17–27) Spiriferellina sp. indet.: (17) internal mold of ventral valve, NIGP 174035; (18, 19) internal mold of ventral valve and its latex cast, NIGP 174036; (20–22) internal mold of ventral valve, its latex cast, and external mold of ventral valve, NIGP 174037; (23) internal mold of ventral valve, NIGP 174038; (24, 25) internal mold of dorsal valve and detail of hinge area of its latex cast, NIGP 174039, (26, 27) external molds of dorsal valve, NIGP 174040, 174041. Scale bars = 5 mm, unless otherwise labeled.
Materials
Registered specimens: one external mold of dorsal valve and its latex cast (NIGP 174025).
Remarks
This incomplete external mold of a dorsal valve, 15.8 mm wide and > 12.9 mm, probably indicates the presence of Phricodothyris in view of its subcircular outline, developed growth lamellae, and especially distinct biramous spines. In the present specimen, two rows of biramous spines are clearly arranged on the anterior part of the concentric lamellae (Fig. 11.3). A similar spine pattern occurs in Phricodothyris attenuata Reed, Reference Reed1944 reported from the Wordian of Khao Phrik, southern Thailand (Waterhouse and Piyasin, Reference Waterhouse and Piyasin1970, p. 157, pl. 26, figs. 7–18), which is also characterized by two or three concentric rows of fine spines lying over the anterior face of the growth lamellae. However, the preservation is not good enough to show whether those spines were double-barreled. Additionally, the concentric lamellae of Phricodothyris attenuata are much denser than those of the present species.
Order Spiriferinida Ivanova, Reference Ivanova1972
Family Reticulariinidae Waterhouse, Reference Waterhouse1975
Genus Reticulariina Fredericks, Reference Fredericks1916
Type species
Spirifer spinosus Norwood and Pratten, Reference Norwood and Pratten1855 from the Carboniferous of the western states, USA.
Remarks
Reticulariina can be easily differentiated from allied genera by its exterior with numerous macroscopic hollow spines, which is totally different from the tiny spinules distributed on the concentric lamellae. In addition, it differs from Paraspiriferina Reed, Reference Reed1944 by its more transverse outline, larger size, and irregularly spaced growth laminae. Crenispirifer Stehli, Reference Stehli1954 can be distinguished by its higher, more angular plications and its less transverse, alate shell.
Reticulariina cf. Reticulariina atava Chronic in Newell et al., Reference Newell, Chronic and Roberts1949
Figure 11.4–11.10
Occurrence
Units 1, 2, 6, and 7, Taungnyo Group, Zwekabin Range, Myanmar.
Description
Shell medium to large, 28.6–41.3 mm wide, 21.1–32.1 mm long, widest at hinge, subequally biconvex in profile, transversely subtrigonal in outline; cardinal extremities angular. Ventral valve moderately convex; beak short, slightly curved; interarea low triangular; delthyrium narrowly wedge-shaped; sulcus originating from umbo, V-shaped, relatively deep, extended into angular tongue; six or seven pairs of plicae on each flank, low, with crests subangular to rounded, interspaces even wider than plicae; pronounced growth lamellae unevenly arranged, ~0.5–1.5 mm apart, well-developed anteriorly; suberect hollow spines regularly located at each side of plicae apex and lateral slopes of sulcus, directing posterior side obliquely; spine bases ~0.5 mm long. Dorsal valve moderately convex; beak very low, broad; fold moderately high, widening forward significantly; 5–7 pairs of low lateral plicae with rounded crests; growth lamellae moderately strong, usually not numerous; hollow spines radially distributed, usually along crest of plicae. Shell substance regularly punctate. Ventral interior with short teeth supported by slightly divergent dental adminicula; median septum mostly covered.
Materials
Registered specimens: one external mold of ventral valve, one incomplete external mold of ventral valve and its latex cast, one external mold of dorsal valve resting inside internal mold of ventral valve and their latex casts, one internal mold of ventral valve resting against external mold of dorsal valve (NIGP 174026–174029).
Remarks
The present species is very similar to some large specimens of Reticulariina atava Chronic in Newell et al., Reference Newell, Chronic and Roberts1949 (p. 106, pl. 20, figs. 2–13) in view of its large size, relatively numerous plicae on the flanks, and the tongue-like sulcus anteriorly (except for its much narrower plications). Reticulariina spinosa (Norwood and Pratten, Reference Norwood and Pratten1855) shares a similar appearance with these Myanmar specimens, but it is only half the size of the latter. Many species of Reticulariina were erected by Cooper and Grant (Reference Cooper and Grant1976) based on collections from the Glass Mountains of western Texas. Among them, Reticulariina powwowensis Cooper and Grant, Reference Cooper and Grant1976 is closest to the present species but differs by its fewer but more angular plicae on each flank and its smaller size.
Family Paraspiriferinidae Cooper and Grant, Reference Cooper and Grant1976
Genus Yaonoiella Waterhouse, Reference Waterhouse1983b
Type species
Yaonoiella mantajiti Waterhouse, Reference Waterhouse1983b from the Kungurian of southern Thailand.
Remarks
The type species of this genus was originally identified as ?Paraspiriferina sp. indet. by Waterhouse (Reference Waterhouse1981, p. 114, pl. 32, fig.1, pl. 33, figs. 5–7), but was later reassigned to Yaonoiella mantajiti mainly based on its narrow fold and sulcus, and much fewer subrounded plicae on the lateral slopes. The present genus is mainly distinguished from Spiriferellina Fredericks, Reference Fredericks1924 by its much lower and rounded plications and regularly spaced growth lamellae.
Yaonoiella mantajiti Waterhouse, Reference Waterhouse1983b
Figure 11.11–11.16
- Reference Waterhouse1981
?Paraspiriferina sp. indet. Waterhouse, p. 114, pl. 32, fig. 1, pl. 33, figs. 5–7.
- Reference Waterhouse1983b
Yaonoiella mantajiti Waterhouse, p. 143.
Holotype
TBR 358, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand (Waterhouse, Reference Waterhouse1981, pl. 32, fig. 1, pl. 33, fig. 7).
Occurrence
Units 2, 3, and 5, Taungnyo Group, Zwekabin Range, Myanmar; Ko Yao Noi Formation, upper Kaeng Krachan Group, Ko Yao Noi, southern Thailand.
Description
Shell small, 9.9–20.1 mm wide, 8.2–11 mm long, transversely subelliptical to rounded in outline; greatest width anterior to hinge or approximately at midlength; cardinal extremities well rounded. Ventral umbo moderately prominent; sulcus narrow, moderately deep; only three pairs of low plicae on lateral slopes with rounded crests, with interspaces as wide as plicae; concentric lamellae densely and regularly spaced, overlapping imbricately; 1–3 rows of spinules arranged between growth lamellae. Dorsal umbo low; fold relatively low and wide in small specimens, becoming higher and narrower in larger ones; two or three pairs of plicae spaced on dorsal flanks, low and simple, with rounded crests; growth lamellae densely spaced, fairly regular, five or six per mm. Shell substance densely punctate.
Ventral interior with short teeth supported by slightly divergent dental adminicular; medium septum long, reaching approximately to midvalve. Dorsal interior with knoblike cardinal process; socket plates subhorizontal, supported by short dorsal adminicula.
Materials
Registered specimens: one internal mold of ventral valve, one latex cast of ventral exterior, two internal molds of dorsal valve, and one external mold of dorsal valve (NIGP 174030–174034).
Remarks
Yaonoiella mantajiti is characterized by small subrounded shells with three pairs of plicae on each flank, and closely, regularly spaced growth laminae bearing a few rows of fine spinules. Therefore, our specimens are included in this species based on the well-matched features. Yaonoiella himalayanica (Waterhouse, Reference Waterhouse1983b), the only other species of the genus, was reported from the Senja Formation of northwestern Nepal (Waterhouse, Reference Waterhouse1983b), and shares similar micro-ornaments but has a more transverse outline and one more pair of plicae.
Family Spiriferellinidae Ivanova, Reference Ivanova1972
Genus Spiriferellina Fredericks, Reference Fredericks1924
Type species
Terebratulites cristatus von Schlotheim, Reference Schlotheim1816 from the Lopingian of Thuringia, Germany.
Remarks
The syntype and lectotype specimens of the type species from the Zechstein of Thuringia, Germany were redescribed and refigured in detail by Campbell (Reference Campbell1959). The entire shell surface of most examined specimens is covered with fine granules (or pustules) that appear to coincide in position with the outer ends of the punctae, although they are suggested as an original surface feature rather than the infill of punctae. It is quite difficult to differentiate the pustules and the infill of punctae in the vast majority of specimens; the same is true for our specimens.
Spiriferellina sp. indet.
Figure 11.17–11.27
Occurrence
Units 1–3, 5 and 6, Taungnyo Group, Zwekabin Range, Myanmar.
Description
Shell 21.3–34.8 mm wide, 13.3–17.6 mm long, with maximum width at hinge or slightly anterior to hinge, subequally biconvex in profile, transversely subtrigonal to subquadrate in outline; cardinal extremities subangular to rounded. Ventral valve moderately convex; beak slightly extended over hinge line, somewhat incurved; umbonal region relatively inflated; sulcus commencing from umbo with gently concave floor and steep sides, gradually widening and deepening forward, with 25° angle; approximately six pairs of plicae on each flank; crests subangular to rounded; interspaces even wider than plicae, inner boundary plicae prominently raised, generally weakening laterally; pronounced growth lamellae unevenly spaced, ~0.6–1.6 mm apart, becoming conspicuous anteriorly; shell covered by close, numerous punctae, approximately five per mm, somewhat irregularly spaced. Dorsal valve gently to moderately convex; beak very broad, low; fold high with rounded crests, relatively wide anteriorly; six or seven pairs of rounded lateral plicae; patterns of growth lamellae and punctae similar to those of ventral valve. Ventral interior with short teeth supported by subparallel dental adminicular; medium septum relatively high, reaching approximately to midvalve. Dorsal interior with two widely divergent sockets formed by strong socket ridges; cardinal process knoblike, composed of tiny parallel platelets; hinge plates growing from socket ridges, broad, curved toward midline, joined to base of cardinal process; crural plates not observed.
Materials
Registered specimens: four internal molds of ventral valve and two of their latex casts, one external mold of ventral valve, one internal mold of dorsal valve and its latex cast, and two external molds of dorsal valves (NIGP 174035–174041).
Remarks
These specimens can be basically assigned to Spiriferellina owing to their transverse outline but without extended hinge ends, smooth or pustulose shell surface except for punctae and relatively weak and irregularly spaced growth laminae. The only exception is that this species has relatively numerous costae. This species is easily differentiated from other species of Spiriferellina by its moderately large size, numerous narrow plicae, and relatively wide fold. Spiriferellina disparata Waterhouse, Reference Waterhouse1987 shares similar size and plications with the present species but differs in its high, narrow fold and very high interarea.
Acknowledgments
The brachiopod specimens studied here were collected during two field trips to Hpa-an Township, Kayin State, Myanmar, by official invitation from the Myanmar Geosciences Society (MGS) in accordance with the Memorandum of Understanding signed between the MGS and Nanjing Institute of Geology and Palaeontology (NIGP), Chinese Academy of Sciences (CAS). The field trips were carried out within officially approved areas in Myanmar, accompanied by two Burmese coauthors and some members of the MGS, with no commercial purpose. We thank M.F. Ridd, W.H. He, and journal editor J.S. Jin for providing very helpful comments to improve the manuscript. This work was funded by the National Science Foundation of China (grant nos. 91855205 and 91955201), Second Tibetan Plateau Scientific Expedition and Research (no. 2019QZKK0706) and Strategic Priority Research Program (B) of the Chinese Academy of Sciences (grant no. XDB26000000).















