Introduction
Since its description based on specimens from the Lower Devonian (Emsian) of the Bokkeveld Group of South Africa, the homalonotid trilobite Burmeisteria Salter, Reference Salter1865 has been considered a typical taxon of the upper Silurian to Middle Devonian of southwestern Gondwanan basins. In South America, Burmeisteria was first recognized by Clarke (Reference Clarke1913) in a study focused on the Devonian of Brazil. In fact, species of this genus (together with the endemic family Calmoniidae) contributed to the notion of an “austral fauna” put forward by Clarke (Reference Clarke1913), which was a precursor of the Malvinokaffric biogeographic province proposed by Richter and Richter (Reference Richter and Richter1942).
Recent revisions mainly include taxonomic reappraisals of closely allied genera including Homalonotus König, Reference König1825; Trimerus Green, Reference Green1832; Digonus Gürich, Reference Gürich1909; and Dipleura Green, Reference Green1832 (see Sandford, Reference Sandford2005; Simões et al., Reference Simões, Leme and Soares2009). However, occurrences of Burmeisteria from Argentina have been largely neglected in these revisions, because published data consist of a few mentions of its presence (see Castellaro, Reference Castellaro1966; Vaccari et al., Reference Vaccari, Waisfeld and Edgecombe1994). In this paper we present new data on homalonotids in the Argentine Precordillera, one of South America's main fossiliferous Lower Devonian basins. We provide information in order to clarify the taxonomy and geographic and stratigraphic distribution of Burmesteria. In addition, we offer some new data and interpretations of the structure and function of the carapace.
Geological and stratigraphic setting
The studied Devonian sedimentary succession corresponds to the Precordillera Basin of Argentina, which is closely allied to a number of other coeval marine basins from southwestern Gondwana. These Devonian basins, which occur in South America, South Africa, and Antarctica, document cold-water, siliciclastic depositional settings characterized by endemism in trilobites and the near absence of typical Paleozoic fossil groups such as graptolites, conodonts, and goniatites. These features define the Malvinokaffric Realm (Richter and Richter, Reference Richter and Richter1942), one of the major marine biogeographic regions during the Devonian.
Our samples come from the Lower Devonian Talacasto Formation (Padula et al., Reference Padula, Rolleri, Mingramm, Criado Roque, Flores and Baldis1967) in San Juan Province, central-western Argentina (Fig. 1). This unit exhibits dark, argillaceous, muddy basal strata, which progress upwards to sandstone at the top of the succession. It corresponds to a muddy shelf depositional system developed during a highstand (Astini, Reference Astini1991), and overlies the (mainly) upper Silurian shelf system of the Los Espejos Formation. The Lower to Middle Devonian Punta Negra Formation lies above the Talacasto Formation. Because classical biostratigraphically useful fossil groups are unavailable, as in other closely related Lower–Middle Devonian Malvinokaffric basins, biostratigraphic calibrations are based on brachiopod and palynological data (Benedetto et al., Reference Benedetto, Racheboeuf, Herrera, Brussa and Toro1992; Herrera, Reference Herrera1993, Reference Herrera1995a, Reference Herrera and Racheboeufb; Le Herissé et al., Reference Le Hérissé, Rubinstein and Steemans1997; Herrera and Bustos, Reference Herrera and Bustos2001; García-Muro et al., Reference García-Muro, Rubinstein and Steemans2014, Reference García-Muro, Rubinstein and Rustán2017, Reference García-Muro, Rubinstein, Rustán and Steemans2018). The base of the Talacasto Formation is early (but not the earliest) Lochkovian throughout the entire basin (Fig. 2), while the top is diachronous and progressively younger from south to north (Racheboeuf and Herrera, Reference Racheboeuf and Herrera1994; Herrera and Bustos, Reference Herrera and Bustos2001). The uppermost levels of the type section of Talacasto Creek include a distinctive, ochre-colored, nodule-bearing marker horizon identified by Keidel (Reference Keidel1921) and Astini (Reference Astini1991). The strata overlying this marker horizon were considered to be early Emsian in age or younger (Herrera and Bustos, Reference Herrera and Bustos2001), but recent palynological information suggests they might be Pragian (García Muro et al., Reference García-Muro, Rubinstein and Rustán2017, Reference García-Muro, Rubinstein, Rustán and Steemans2018).
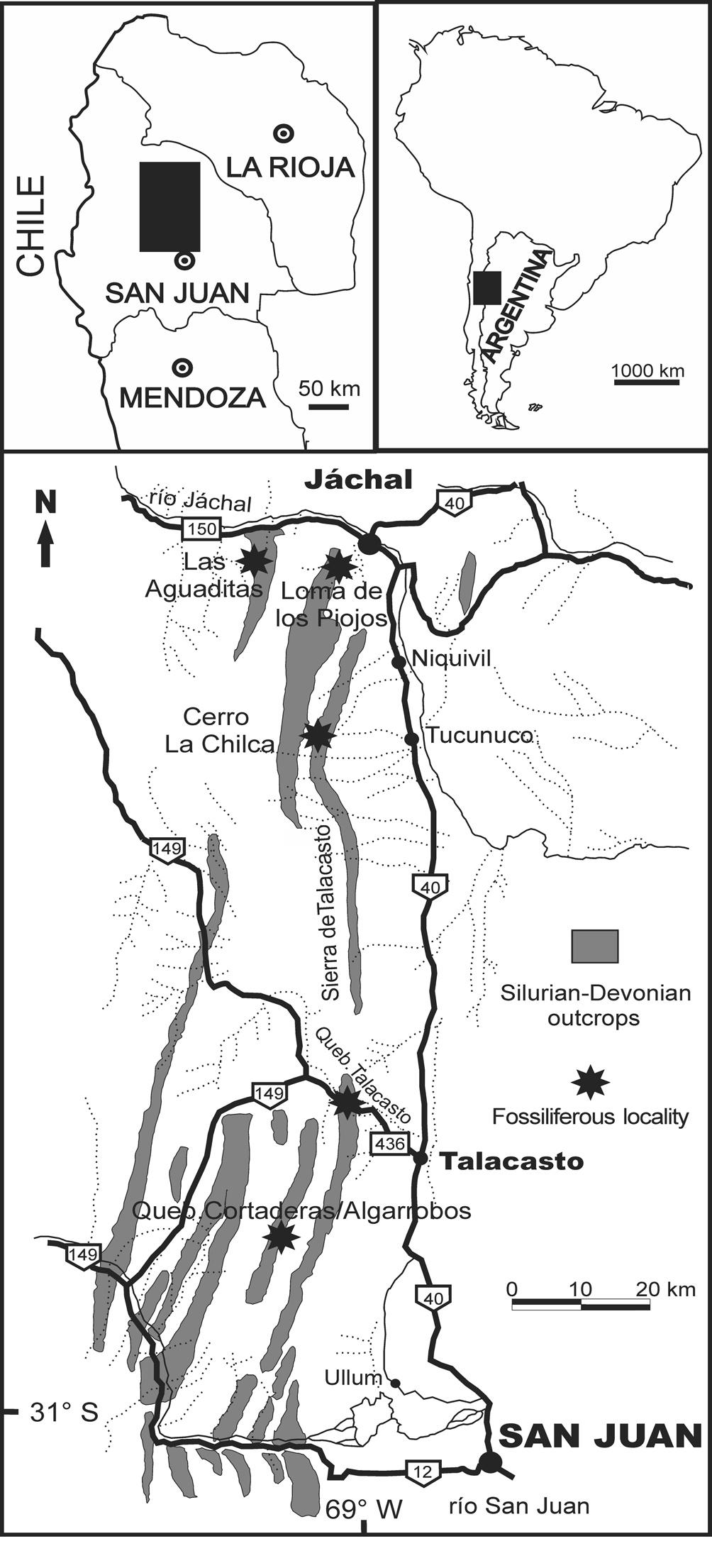
Figure 1. Map of the studied area in the Central Precordillera of the San Juan Province (Argentina), showing Silurian–Devonian outcrops between San Juan and Jáchal cities and the main fossiliferous localities.

Figure 2. Stratigraphic sections and intervals where Burmeisteria occurs. Excluding a couple of Lochkovian records in the Precordillera Basin, this trilobite is virtually restricted to sandy Pragian layers underlying the top of the Keidel's (Reference Keidel1921) marker horizon. Selected sections included in the figure are those typical from the southern (Talacasto) and northern (Loma de los Piojos) areas in the study region.
Burmeisteria has not been recorded above Keidel's marker (Fig. 2). Abundant sclerites derive from Keidel's bed itself, and from the underlying Pragian interval of greenish-gray to bluish micaceous wackestone (the bluish sandstone member referred to by Espisúa, Reference Espisúa1968). Just one specimen was recorded in the Lochkovian greenish-dark mudstones of the lowermost stratigraphic interval (at the Quebrada de los Algarrobos section nearly 30 km to the south of the Talacasto Formation type locality). It comes from nearly 20 m above the base (around 31°11'21.4"S, 68°49'18.5"W), ~50 m below the level from which Carrera et al. (Reference Carrera, Montoya, Rustán and Halpern2013) reported branched corals in life position.
The surveyed fossiliferous sections include, from south to north (Fig. 1): Los Algarrobos (main collecting site around 31°11'36"S, 68°49'32.3"W), Talacasto, La Chilca (main collecting site around 30°35'31.9"S, 68°48'36.9"W), Río de las Casitas, and Loma de los Piojos (main collecting site around 30°17'31.2"S, 68°46'39.5"W). Additional specimens derive from outcrops at Cordón del Peñón (nearly 20 km to the west of La Chilca section) and Pasleam (nearly 15 km to the northwest of Jáchal city), but exact locations are uncertain. Further details of the stratigraphy and correlation of each section can be found in Vaccari et al. (Reference Vaccari, Waisfeld and Edgecombe1994), Herrera and Bustos (Reference Herrera and Bustos2001), Carrera et al. (Reference Carrera, Montoya, Rustán and Halpern2013, Reference Carrera, Ernst and Rustán2019), and Sterren et al. (Reference Sterren, Rustán and Salas2015).
Repositories and institutional abbreviations
The specimens are housed under the prefix CEGH-UNC in the paleontological collection of Centro de Investigaciones Paleobiológicas (CIPAL), in the Centro de Investigaciones en Ciencias de la Tierra (CICTERRA), Universidad Nacional de Córdoba, Argentina. DGM, Departamento Nacional de Produção Mineral, Rio de Janeiro, Brazil. SU, Geological Collections, Stellenbosch University, South Africa.
Systematic paleontology
Order Phacopida Salter, Reference Salter1864
Suborder Calymenina Swinnerton, Reference Swinnerton1915
Superfamily Calymenoidea Burmeister, Reference Burmeister1843
Family Homalonotidae Chapman, Reference Chapman1890
Genus Burmeisteria Salter, Reference Salter1865
Type species
Homalonotus Herschelii Murchison, Reference Murchison1839, p. 652, pl. 7bis (fig. 2), Gydo Formation, Lower Devonian (Emsian), Bokkeveld beds, South Africa, by original designation.
Burmeisteria herschelii (Murchison, Reference Murchison1839)
Figures 3, 4
Reference Murchison1839 Homalonotus Herschelii Murchison, p 652, pl. 7bis, fig. 2.
Reference Salter1856 Homalonotus herschelii; Salter, p. 215, pl. 24, figs. 1–8.
Reference Salter1865 Homalonotus (Burmeisteria) herschelii; Salter, p. 105.
Reference Frech1897 Homalonotus perarmatus; Frech, p. 218.
Reference Lake1904 Homalonotus herschelii; Lake, p. 214, pl. 26, figs. 1–3.
Reference Lake1904 Homalonotus quernus; Lake, p. 216, pl. 27, fig. 1.
Reference Lake1904 Homalonotus colossus; Lake, p. 216, pl. 28, figs. 1–3.
Reference Schwarz1906 Homalonotus herschelii; Schwarz, p. 383, pl. 8, fig. 8, pl. 9, fig. 3.
Reference Schwarz1906 Homalonotus herschelii Murchison Salter var?; Schwarz, p. 383, pl. 8, fig. 8.
Reference Schwarz1906 Homalonotus horridus; Schwarz, p. 385, pl. 9, fig. 1.
Reference Schwarz1906 Homalonotus agrestis; Schwarz, p. 386, pl. 9, fig. 2.
Reference Schwarz1906 Homalonotus hippocampus; Schwarz, p. 388, pl. 9, fig 5.
Reference Schwarz1906 Homalonotus lex; Schwarz, p. 389, pl. 9, fig. 4.
Reference Clarke1913 Homalonotus herscheli; Clarke, p. 93, pl. 3, figs. 1–4.
Reference Reed1925 Homalonotus (Burmeisteria) herschelii; Reed, p. 163.
Reference Reed1925 Homalonotus (Burmeisteria) herschelii var. rectisuturalis Reed, p. 167, pl. 10, figs. 3, 7.
Reference Reed1925 Homalonotus (Burmeisteria) herschelii var. colossus; Reed, p. 169.
Reference Reed1925 Homalonotus (Burmeisteria) herschelii var. fusiformis Reed, p. 171.
Reference Reed1925 Homalonotus (Burmeisteria) herschelii var. grahami Reed, p. 172.
Reference Reed1925 Homalonotus (Burmeisteria) herschelii var. bituberculata Reed, p. 173, pl. 10, fig. 2.
Reference Reed1925 Homalonotus (Burmeisteria) herschelii var. perarmata; Reed, p. 175.
Reference Reed1925 Homalonotus (Burmeisteria) quernus; Reed, p. 181, pl. 9, fig. 13, pl. 10, fig. 1.
Reference Reed1925 Homalonotus (Burmeisteria?) hippocampus; Reed, p. 183.
Reference Reed1925 Homalonotus (Burmeisteria) sp. Reed, p. 184.
Reference Sdzuy1957 Burmeisteria hippocampus; Sdzuy, p. 283, fig. 1.
Reference Saul, Doumani, Boardman, Rowell, Boucot, Johnson, Lee-McAlester, Saul, Fisher and Miles1965 Burmeisteria herschelii; Saul, p. 271.
Reference Braniša1965 Burmeisteria herschelii; Braniša, p. 84, pl. 10, figs. 1, 4.
Reference Braniša1965 Burmeisteria cf. herschelii; Braniša, p. 84, pl. 11, fig. 6.
Reference Wolfart, Wolfart and Voges1968 Burmeisteria (Burmeisteria?) sp. Wolfart in Wolfart and Voges, p. 61.
Reference Pek and Vaněk1991 Burmeisteria verrucosa Pek and Vaněk, p. 80.
Reference Soares, Simões and Leme2008 Burmeisteria herschelii; Soares et al., p. 124, pl. 4, figs. G, H.
Reference Simões, Leme and Soares2009 Burmeisteria herschelii; Simões et al., p. 32, pl. 3, fig. A, pl. 4, figs. G, H, pl. 6, fig. A.

Figure 3. (1–15) Burmeisteria herschelii (Murchison, Reference Murchison1839), cephalic morphology. All specimens from Pragian nodules of the middle-upper part of the Talacasto Formation at Cerro la Chilca Section. (1–6) CORD-PZ 8592, partially preserved cephalon; (1) dorsal view; (2) frontal view; (3) lateral view; (4) ventral view; (5) rostral plate in ventral view; (6) detail of the anteroventral process of the rostral plate. (7–12) CEGH-UNC 12756, partially preserved cephalon; (7) dorsal view; (8) frontal view; (9) lateral view; (10) detail of preservation, where carapace is intact the external surface exhibits tiny pores, where decorticated granules (filling of poral channels) can be observed; (11) dorso-anterolateral view; (12) detail of the preservation. (13) CEGH-UNC 12758, partially preserved cephalon, dorsal view. (14) CEGH-UNC 24251, partially preserved cephalon, dorsal view, note tubercles in the occipital ring. (15) CEGH-UNC 12757, partially preserved cephalon, dorsal view. Scale bars = 5 mm.

Figure 4. Burmeisteria herschelii (Murchison, Reference Murchison1839), thoracic and pygidial morphology. (1–10) Specimens from Pragian nodules of the middle upper part of the Talacasto Formation at the Cerro La Chilca Section. (1–3) CEGH-UNC 24253, partially preserved pygidium; (1) dorsal view; (2) posterior view; (3) lateral view. (4–6) CEGH-UNC12754, partially preserved pygidium; (4) dorsal view; (5) posterior view; (6) lateral view. (7–10) CEGH-UNC 24260, partially preserved pygidium; (7) lateral view; (8) dorsal view; (9) detail, lateral view; (10) detail of preservation, where carapace is intact the external surface exhibits tiny pores, where decorticated granules (filling of poral channels) can be observed. (11, 13) CEGH-UNC 24246, thoracic segments with partially preserved carapace, Loma de los Piojos section, immediately below the Keidel's marker horizon, Pragian; (11) lateral view; (13) dorsal view. (12) CEGH-UNC 12752, internal mold of an articulated thorax partially preserved, dorsal view. Scale bars = 5 mm.
Type specimen
Homalonotus Herschelii, thoracopygidium, Gydo Formation, Lower Devonian (Emsian), Bokkeveld beds, South Africa, by original designation (Murchison, Reference Murchison1839, p. 652, pl. 7bis, fig. 2).
Diagnosis
Burmeisteria reaching large sizes (~30 cm) as mature holaspids; with strong tubercles and spines irregularly distributed on the cephalon (particularly on the posterior border) and asymmetrically located, but tending to be ordered in longitudinal rows along the thorax and pygidium; glabella somewhat variable in shape from approximately trapezoidal to distinctly urceolate; glabellar furrows wide (exsag.) and variably impressed from faint to well-defined, giving a lobate aspect to the glabella; pygidium triangular and elongated in dorsal view (sag.), with up to 16–17 axial rings.
Occurrence
Mainly the middle, sandy part of the Talacasto Formation (Pragian) at Talacasto, La Chilca, Pasleam, Sierra del Peñón, and Loma de los Piojos sections, San Juan Province.
Description
A full description was provided by Cooper (Reference Cooper1982, p. 18). A discussion of the external characters of the carapace is given below, under the treatment of B. notica.
Nomenclatural note
Although the original spelling by Murchison (Reference Murchison1839, p. 652) was “Homalonotus Herschelii,” some subsequent works have used herscheli, which is a case of “incorrect subsequent spelling.” According to ICZN art. 33, the original spelling must be preserved, except in the case of prevailing use. Because recent publications have not stabilized the species name (e.g., Carvalho, Reference Carvalho2005; Sandford, Reference Sandford2005; Soares et al., Reference Soares, Simões and Leme2008; Simões et al., Reference Simões, Leme and Soares2009), the original spelling, Burmeisteria herschelii, is preferred herein.
Remarks
Although a number of studies have dealt with Burmeisteria since Murchison (Reference Murchison1839), a formal diagnosis of B. herschelii has not been published, as far as we are aware. Recognition of the species is currently based on a set of a few characters mentioned in discussions by different authors, pending a revised diagnosis. The species can be reliably identified using the unique combination of characters listed above in our proposed diagnosis.
Burmeisteria herschelii has been considered to be strikingly polymorphic (Reed, Reference Reed1925; Cooper, Reference Cooper1982; Sandford, Reference Sandford2005). The variability is noticeable in the degree of expression of the glabellar furrows, curvature of the cephalic axial furrows (which define a more or less urceolate glabellar outline), rostral suture, expression of the paraglabelar areas, course of the cephalic sutures (slightly) and, fundamentally, the size, abundance, and distribution pattern of tubercles and spines (see Cooper, Reference Cooper1982, p. 18).
In combination with a strongly plesiomorphic bauplan, the remarkable degree of intraspecific variability in this trilobite complicates the taxonomic interpretations of a number of closely related species. Burmeisteria limabambaensis Pek and Vaněk, Reference Pek and Vaněk1991 was erected on the basis of a couple of incomplete cephala illustrated by Braniša (Reference Braniša1965, pl. 10, figs. 8, 9) from the upper member of the Belén Formation (late Emsian? to Givetian?), at the Limabamba section, Bolivia. Pek and Vaněk's diagnosis includes characters, such as three shallow glabellar furrows, that occur widely in the genus, including B. herschelii (Harrington et al., Reference Harrington and Moore1959, p. O460; Saul, Reference Saul1967, p. 1135; Cooper, Reference Cooper1982, p. 21; Sandford, Reference Sandford2005, p. 23).
The preglabellar field was described as wide (sag.), but measurements from Braniša's (Reference Braniša1965) figures show that it comprises only 12% of cephalic length, so it is actually shorter than in species such as B. notica (25% of cephalic length, according to Cooper, Reference Cooper1982, p. 26). The course of the cephalic sutures is difficult to discern in Braniša's (Reference Braniša1965) illustrations, but does not seem to differ significantly from that of B. herschelii. The palpebral lobes and eyes of B. limabambaensis are as variable in morphology, size, and position as they are in B. herschelii (see illustrations in Cooper, Reference Cooper1982). The cephalic posterior border was described as “convex just as the occipital ring” (Pek and Vaněk, Reference Pek and Vaněk1991, p. 79), but this cannot be properly evaluated from illustrations. The absence of an occipital node is actually shared with B. herschelii. Braniša (Reference Braniša1965) claimed that, in both type specimens, the librigena is wider transversely and the lateral border is more prominent than in B. hershelii, but these features are not clearly visible in his illustrations. A fine, even tuberculation seems to be a real character that might be used to discriminate between these species, but was not considered in the diagnosis given by Pek and Vaněk (Reference Pek and Vaněk1991).
Burmeisteria boliviana Pek and Vaněk, Reference Pek and Vaněk1991 was based on two fragmentary, deeply decorticated, cephala (pl. 11, figs. 1, 2) from the upper member of the Belén Formation, at the Chacoma section, Bolivia. Pek and Vaněk's (Reference Pek and Vaněk1991) diagnosis mentioned a urceolate glabella with three pairs of glabellar furrows, of which the preoccipital one (S1) is progressively wider towards the median plane, curving posteriorly. However, these characters are typical of the entire genus and have been reported repeatedly in B. herschelii (Harrington et al., Reference Harrington and Moore1959, p. O460; Cooper, Reference Cooper1982, p. 19). The occipital ring was described as “smooth and bounded by almost indistinct axial furrows” in the diagnosis, but specimens that undoubtedly belong to B. herschelii also display these character states (see Cooper, Reference Cooper1982, figs. 27 A, 28 A, 28 B). The relatively straight course of the anterior branches of the cephalic sutures of B. boliviana is within the range of variability exhibited by B. herschelii (see Cooper, Reference Cooper1982, figs. 14, 17 A, 18 F, 21, 28). The posterior sutural branches are not oriented towards the posterior border, as stated in the diagnosis, but toward the lateral border and then to the genal angle (thus defining a typical gonatoparian suture). Further details of the librigena and posterior and lateral borders are difficult to evaluate from available illustrations.
Since the diagnoses of B. limabambaensis and B. boliviana neither enumerate apormorphies nor constitute a unique combination of characters, they are of no use for recognizing species. The type specimens are poorly preserved and do not display tubercles or spine bases. It is possible that both B. limabambaensis and B. boliviana are junior synonyms of B. herschelii, but the issue merits a revision of the original materials in order to eventually amend the diagnosis. For the moment, we consider that the taxonomic identity of both species cannot be determined from the primary types, and treat them as nomina dubia (ICZN, art. 75.5).
Burmeisteria verrucosa Pek and Vaněk, Reference Pek and Vaněk1991 was based on two fragmentary pygidia illustrated by Braniša (Reference Braniša1965, pl. 10, figs. 1, 4) from the Gamoneda Formation (Pragian–Emsian), at the Gamoneda-Curuyo section, Bolivia. In their comparison with B. herschelii, Pek and Vaněk pointed out that the axis of B. verrucosa is anteriorly wider (tr.) and constricted in the posterior third; the pleural ribs also wider (exsag.) and more prominent, and spines and granules stronger and more abundant. Nevertheless, a comprehensive revision by Cooper (Reference Cooper1982), who reillustrated most of the type material of a number of varieties and synonyms proposed since the mid-19th century, shows that all of the characters listed by Pek and Vaněk (Reference Pek and Vaněk1991) are observable in B. herschelii. In particular, Cooper's (Reference Cooper1982) figures 15 C, 19 B, and 27 B showed that the characters attributed to B. verrucosa as putative differences from B. herschelii do not justify taxonomic separation. Consequently, B. verrucosa should be considered a subjective junior synonym of B. herschelii.
Homalonotus magnus Méndez-Alzola, Reference Méndez-Alzola1938, from the Emsian of the El Cordobés Formation, Durazno Department, Uruguay, was based on a single articulated specimen, mainly preserved as an internal mold, lacking the pygidium (p. 54, pl. 12, fig. 5, pl. 13, fig. 1). The species was diagnosed by its large size (18 cm of sagittal length without the pygidium, and 12.6 cm of transverse width in the posterior part of the cephalon), by the absence of well-impressed glabellar lobation, and by a lack of strong tuberculation or spinosity. Later, this species was referred to Dipleura by Sprechmann et al. (Reference Sprechmann, Montaña, Gaucher and Bossi1993, p. 34). However, the large size of the exoskeleton and the presence of tubercles or spine bases in some thoracic segments are strictly similar to B. herschelii, in spite of some deformation. This evidence, along with the urceolated glabella, which actually exhibits lobation, suggests that Homalonotus magnus is a junior synonym of B. herschelii.
Burmeisteria notica (Clarke, Reference Clarke1913)
Figures 5–7
Reference Clarke1913 Homalonotus noticus; Clarke, p. 89, pl. 1, figs. 1, 2; pl. 2, figs. 9–13.
Reference Kozłowski1913 Homalonotus sp.; Kozłowski, p. 13, pl. 3, figs. 8, 9.
Reference Kozłowski1923 Homalonotus cf. noticus; Kozłowski, p. 23, pl. 1, fig. 11.
Reference Reed1925 ?Homalonotus (Burmeisteria) herscheli var. rectisuturalis; Reed, p. 168, just specimen SU-C1.
Reference Reed1925 Homalonotus (Digonus) noticus var. africana Reed, p. 184, pl. 10, fig. 4.
Reference Sdzuy1957 Digonus noticus; Sdzuy, p. 279.
Reference Ahlfeld and Braniša1960 Digonus noticus; Ahlfeld and Braniša, p. 60, pl. 6, fig. 13.
Reference Braniša1965 Digonus cf. noticus; Braniša, p. 84, pl. 10, figs. 5–7, pl. 11, fig. 13, pl. 75, fig. 2.
Reference Saul, Doumani, Boardman, Rowell, Boucot, Johnson, Lee-McAlester, Saul, Fisher and Miles1965 Burmeisteria (Digonus) noticus; Saul, p. 271.
Reference Wolfart, Wolfart and Voges1968 Burmeisteria (Digonus) cf. noticus; Wolfart, p. 62.
Reference Sandford2005 Burmeisteria notica; Sandford, p. 23.
Reference Soares, Simões and Leme2008 Digonus noticus; Soares et al., p. 124, pl. 4, fig. B-F.
Reference Simões, Leme and Soares2009 Burmeisteria noticus; Simões et al., p. 32, pl. 3, fig. B, pl. 4, figs. B–F, pl. 6, fig. B.

Figure 5. (1–13) Burmeisteria notica (Clarke, Reference Clarke1913) cephalic morphology. All specimens from Pragian nodules of the middle upper part of the Talacasto Formation at Cerro la Chilca Section. (1–4, 7) CEGH-UNC 24187, partially preserved cephalon; (1) dorsal view; (2) frontal view; (3) lateral view; (4) dorso-anterolateral view; (7) detail of porous dorsal external surface of the carapace. (5, 6) CEGH-UNC 24154, fragmentary cephalon; (5) dorsal view; (6) frontal view. (8–10) CEGH-UNC 24188, Partially preserved cephalon; (8) dorsal view; (9) frontal view; (10) lateral view. (11–13) CEGH-UNC 24164, partially preserved cephalon; (11) dorsal view; (12) frontal view; (13) lateral view. Scale bars = 5 mm.

Figure 6. Burmeisteria notica (Clarke, Reference Clarke1913), pygidial morphology. (1–16) Specimens from Pragian nodules of the middle upper part of the Talacasto Formation at Cerro la Chilca Section. (1–3. 16) CEGH-UNC 24101, partially preserved pygidium; (1) dorsal view; (2) posterior view; (3) lateral view; (16) ventral view. (4–6, 14) CEGH-UNC 24102, Partially preserved pygidium; (4) dorsal view; (5) posterior view; (6) lateral view; (14) ventral view. (7–9, 13) CEGH-UNC 24107, partially preserved pygidium; (7) dorsal view; (8) posterior view; (9) lateral view; (13) ventral view. (10–12) CEGH-UNC 24103, partially preserved pygidium; (10) dorsal view; (11) posterior view; (12) lateral view. (15) CEGH-UNC 24110, ventral view. Scale bars = 5 mm.

Figure 7. Burmeisteria notica (Clarke, Reference Clarke1913), thoracic and pygidial morphology. (1–9) Specimens from Pragian nodules of the middle upper part of the Talacasto Formation at Cerro la Chilca Section. (1, 4) CEGH-UNC 12755; partially preserved toracopygidium; (1) dorsal view; (4) lateral view. (2, 6) CEGH-UNC 12751, almost complete articulated specimen, internal mold; (2) dorsal view; (6) dorsal view. (3, 5) CEGH-UNC 24110, toracopygidium; (3) dorsal view (5) lateral view. (7) CEGH-UNC 24121, partially preserved pygidium, dorsal view. (8, 9) CEGH-UNC 24092, partially preserved pygidium; (8) dorsal view; (9) lateral view. (10, 11, 13, 14) CEGH-UNC 24244, very well-preserved cephalon, Cordón del Peñón, undetermined stratigraphic level, note the external pitted surface of the carapace; (10) dorsal view; (11) detail of the carapace; (13) dorsal view; (14) fixigena, dorsal view. (12) CEGH-UNC 24184, exfoliated pygidium, note granules (filling of poral channels) corresponding to external pits or pores. (15) CEGH-UNC 24184, detail of poral filling (granules), where the most external layer of the carapace is lost. Scale bars = 5 mm.
Type specimens
Homalonotus noticus, Ponta Grossa Formation, Lower Devonian (Pragian), Ponta Grossa, Paraná, Brazil, by original designation (Clarke, Reference Clarke1913, p. 89, pl. 1, figs. 1, 2, pl. 2, figs. 1–13). Lectotype, almost complete articulated specimen, DGM, 55- I (Carvalho and Ponciano, Reference Carvalho and Ponciano2015).
Diagnosis
Burmeisteria with a longitudinal (adaxial) row of small thoracic tubercles adjacent to the axial furrows; subtrapezoidal to approximately urceolated glabella, with inconspicuous to weakly impressed glabellar furrows; triangular pygidium tending to be equilateral (not clearly sagitally elongated); abruptly acuminate posteriorly, with up to 14–15 axial rings.
Occurrence
Middle part of the Talacasto Formation, at Talacasto, La Chilca, Pasleam, Cordón del Peñón, and Loma de los Piojos sections, San Juan Province.
Description
Cooper (Reference Cooper1982, p. 36) pointed out that similarity and high variability largely preclude a description of B. notica, which is significantly different from that of B. herschelii. However, we use abundant, well-preserved material of B. notica to update and improve Clarke's (Reference Clarke1913) original description.
Elongated body (sag.), length (sag.)/width (tr.) ratio of 2.5, slender and slightly subtriangular shape (Fig. 7.6), defined by cephalon that is wider (tr.) than pygidium, and thoracic segments that are progressively narrower (tr.) posteriorly. Transverse profile strong and evenly convex dorsally (Fig 6.2, 6.5, 6.8, 6.11). Smooth, except for two longitudinal rows of delicate tubercles immediately adjacent to the apodemal fossulae of thoracic axial furrows; carapace is covered by pores (Fig. 7.10, 7.11, 7.13, 7.14).
Cephalon of subtriangular outline in dorsal view, wide (tr.) and relatively depressed in frontal view, with anterior margin nearly straight (Fig. 5); length/width ratio of almost 0.9. Frontal area slightly inclined anteriorly, continuing with anterior profile of glabella in lateral view. Anterior margin formed the connective suture, due to dorsal displacement of rostral plate, variable from slightly concave to nearly transverse in dorsal view. Anterior border not distinct. Anterior border furrow not identifiable, apparently corresponding to the most concave part anteriorly to preglabellar furrow. Preglabellar field subtly concave, and gently inclined anteriorly. Preglabellar furrow slightly convex anteriorly up to approximately straight in dorsal view, short (sag., exsag.) and shallow. Glabella (excluding occipital ring) subrectangular to slightly urceolate/subtrapezoidal, anterolateral corners widely and regularly curved, narrower (tr.) anteriorly (posterior/anterior width radio ~0.7), weakly inflated, length (sag., excluding occipital ring) 65% of cephalic length, and 40% of its width (at level of the occipital ring). Axial furrows approximately straight to slightly concave, wide (tr.) and shallow, narrowing and shallowing backward. SO short (sag., exsag.) and moderately deep, incised, shorter, shallower, and convex forward medially, laterally bounded by small concavities. S1 variably impressed, wide (sag., exsag.) and shallow, curving backwards near sagittal plane. S2 located slightly anteriorly to palpebral lobe, poorly defined (to effaced), in general a gentle depression elongated transversely, tending to curve backwards, shallowest at junction with axial furrows. S3 as subtle exsagittal depression (without reaching axial furrows) in anterior third of glabella, or absent. LO (occipital ring) moderately long (sag., exsag.), longest and convex forward at sagittal plane, moderately short adaxially, lengthening (exsag.) laterally, bounded by indistinct axial furrows, slightly convex dorsally, less convex than posterior part of glabella in lateral view. L1 moderately long (exsag.) reaching up to 30% of sagital length of glabella excluding occipital ring, variably expressed but conspicuous in general. L2 very poorly defined or absent. L3 weakly defined or absent. Anterior section of facial suture nearly straight to slightly sinusoidal, intersecting lateral margin at low angle. Preocular area of fixigena without ocular ridges, with convexity increasing towards interocular region, defining a long and depressed area at anterolateral extreme of glabella. Interocular area of fixigena moderately narrow (tr.) and inclined toward axial furrows. Postocular area of fixigena, steeply inclined (becoming rapidly subvertical) towards lateral border, bearing conspicuous paraglabellar areas, and long and convex region laterally that runs posteromedially between posterior border furrow and depression associated with posterior branch of facial suture. Paraglabellar areas large and conspicuous, slightly asymmetrical drop-shaped, moderately elongated in anteromedial-posterolateral direction, bounded anteriorly by subocular border, laterally by axial furrows towards sagittal plane and posteriorly by posterior border furrow, slightly depressed and with faint ornamentation. Palpebral lobe poorly known. Posterior border furrow long (exsag.) and shallow, widespread behind paraglabellar area, widening and shallowing laterally, then describing a gentle curve posterolaterally. Posterior border moderately long (exsag.), shallower towards genal angle. Genal angle poorly known, rounded in appearance. Librigenal field steeply inclined (becoming nearly subvertical) laterally, moderately narrow (tr.), slightly concave towards lateral border, maximum convexity or inflation surrounding base of eye, defining a subocular border. Subocular border as a wide (tr.) mammiform platform, regularly convex dorsally (except by intersection of depression associated with postocular portion of facial suture), exhibiting a subocular furrow immediately adjacent to base of eye. Relatively small eye, tall and pedunculate, at least as tall as glabella, subrounded to approximately kidney-like in dorsal view. Lateral margin (librigenal) regularly curved. Lateral border recognizable as a wide (tr.) slightly convex band, subparallel to lateral margin. Lateral furrow weakly defined, as a subtle depression subparallel to lateral margin. Posterior section of facial suture proparian, associated with a depression, dorsal portion approximately straight and lateral, one rapidly curved posteriorly, running subparallel to lateral margin before intersecting at low angle.
Rostral plate with anterior part dorsally exposed as extended (tr.) triangular area, having a spine-like projection anteriorly directed (Figs. 5.5, 8). Connective suture visible dorsally, approximately transverse or slightly concave posteriorly, barely narrower (tr.) than anterior margin of cranidium. Spinose to bulbous ventral projection anteroventrally directed, visible dorsally. Ventral portion without material for observation. Hypostome unknown Thorax elongated (described on the basis of specimen CEGH-UNC 12751, except when indicated), with 13 segments, evenly convex in transverse cross section. Thoracic segments moderately short (sag., exsag.). Axial furrows evenly convex laterally, shallow and moderately wide (tr.), narrowing (tr.) and shallowing posteriorly, better defined in posterior part of each segment where apodemal fossula present. Fulcrum inconspicuous and located very close to axial furrow. Axial ring very wide (tr.), up to 70–75% of total thoracic width (tr.), measured at the fourth thoracic segment, slightly convex dorsally in lateral view, approximately twice length (sag.) of articulating half ring, bearing a small tubercle adjacent to each axial furrow. Articulating furrow very short (sag., exsag.) and shallow, crossing axial furrow obliquely in an anteromedial-posterolateral orientation, then continuing in pleural furrow. Articulating half ring moderately short (sag., exsag), and slightly convex in lateral view. Pleural furrow short (exsag.) and shallow, incised, as continuation of the articulating furrow across axial furrow, then entering articulating facet laterally where it becomes delicate trace that curves posteriorly and disappears before reaching pleural margin distally (this trace is anteriorly concave in most anterior thoracic segments and convex to sinusoidal in most posterior ones). Posterior pleural band about twice length (exsag.) of anterior pleural band near the fulcrum, with apodemal fossula at intersection with axial furrow, and faint node posteriorly, in relation to wide (tr.), triangular articulating structure of anterior pleural band of immediately posterior segment. Articulating facet well developed, smooth and flat, posterior border convex or lobate, without posterior truncation. Pleural tip rounded to slightly subquadrate.
Pygidium (description based on several specimens) slightly shorter (sag.) than cephalon, very high anteriorly, with trigonal to elongated triangular shape in dorsal view, with mean ratio length (sag. including articulating half ring)/width (tr.) of nearly 0.98, strongly convex dorsally in posterior view. Axis with anterior width (tr.) ~50–55% of total width (tr.), segmented, indistinct postaxial ridge, slightly more convex dorsally than pleural fields. Up to 15 axial rings (usually between 10 and 14), moderately long (sag., exsag), the first one more noticeable by well-impressed articulating furrow; remaining rings, gradually less conspicuous posteriorly, without tubercles except for very faint ones in a few cases, slightly convex in lateral view. Articulating furrows very shallow and short (sag., exsag.), incised. Axial furrows moderately wide (tr.) and shallow, intersecting articulating furrow of the first segment, approximately straight, and gradually converging backwards. Pleural lobes well developed, strongly inclined (up to subvertical) and broadly convex laterodorsally in posterior view. Articulating facet anterolateral, crossed by the sinuous portion of deeply incised, articulating furrow that curves backwards and dies out before reaching pygidial margin. Posterior border of articulating facet projected backwards as a nearly straight and subhorizontal edge, which gradually becomes fainter posteriorly, constituting the lower level where the pygidial segmentation is noticeable laterally. Nine to 11 (rarely 12) pleural ribs, without conspicuous pleural furrows, and with same number of interpleural furrows, moderately wide (tr.) and shallow, becoming progressively effaced posteriorly and laterally. Pygidial border barely identifiable as nearly smooth band subparallel and adjacent to pygidial margin. Posterior pygidial tip, short, strong and thick, dorsally oriented. Doublure narrow (tr.), narrow furrow (tr.), shallow and incised, shallowing posteriorly yet entering posterior pygidial tip ventrally.
Remarks
The distinction among Burmeisteria notica, B. herschelii, and related species is based on new Argentinian material, published illustrations, and comparisons in the literature, particularly by Cooper (Reference Cooper1982) and Simões et al. (Reference Simões, Leme and Soares2009). Burmeisteria notica has been characterized as devoid of tubercles (Cooper, Reference Cooper1982, p. 26–27; Simões et al., Reference Simões, Leme and Soares2009). However, well-preserved material shows two longitudinal rows of small thoracic tubercles immediately adjacent to the apodemal fossulae of the thoracic axial furrows, adaxially (Fig. 7.1, 7.3, 7.6). This character is clearly observable in fossils from Brazil (Simões et al., Reference Simões, Leme and Soares2009, fig. 3 B, see the left side of most posterior thoracic segments), but is barely perceptible in specimens from South Africa (Cooper, Reference Cooper1982).
Burmeisteria notica has been frequently referred to Digonus Gürich, Reference Gürich1909, either as a subgenus of Burmeisteria or as an independent genus. In this paper we follow the discussions and revised diagnosis of Digonus given by Sandford (Reference Sandford2005, p. 21, 23) and assign the species to Burmeisteria based on the expanded (tr.) glabella at the level of L1, concave cephalic axial furrows, relatively narrow pygidial axis (tr.), well-impressed axial furrows on the first segment of the pygidium, and barely defined pleural ribs.
Kennedy (Reference Kennedy1994) noted that the pygidial doublure of Digonus is often narrower than in Burmeisteria, and bears a narrow groove in the margin. However, the presence of a narrow, furrowed pygidial doublure in our material of B. notica shows that this character does not separate these genera. Homalonotus clarkei Kozłowski, Reference Kozłowski1923 was based on two cephala and two fragmentary pygidia, most likely from the Icla Formation (Pragian–Eifelian) at the Padilla and Tarabuco-Saropaya sections, Padilla-Tarabuco area, Bolivia. The pygidia were assigned to the cephala because “ces deux parties ont été rencontrée dans un mème gisement, où aucune autre espèce de ce genre n`a été trouvée” (Kozłowski, Reference Kozłowski1923, p. 24). In the original work, no holotype was selected, and Wolfart (Reference Wolfart, Wolfart and Voges1968, p. 63) chose one of Kozłowski's (Reference Kozłowski1923, pl. 12, fig. 1) cephala as a lectotype. The species was later assigned variously to Digonus (Wolfart, Reference Wolfart, Wolfart and Voges1968), Trimerus (Tomczykowa, Reference Tomczykowa1975–with doubts), Burmeisteria (Cooper, Reference Cooper1982), and Dipleura (Wenndorf, Reference Wenndorf1990).
Although the name-bearing type of H. clarkei is a cephalon, many discussions (Saul, Reference Saul1967, p. 1134; Cooper, Reference Cooper1982, p. 39; Sandford, Reference Sandford2005, p. 23) were based on the pygidia originally illustrated by Kozłowski (Reference Kozłowski1923), and other specimens later reported by Braniša (Reference Braniša1965, pl. 11, figs. 1, 8, 9, 15), none of which was in organic association with cephala.
These pygidia are triangular, short (sag.), with weakly expressed segmentation (fundamentally on the pleural areas), an only subtly defined axis (with the first axial furrow slightly more evident), and with concave margins towards rear, conferring a remarkable acuminated aspect.
Our collection from the concretionary level of the Cerro La Chilca section, where all the non-tuberculate cephala unquestionably belong to B. notica (Fig. 5), yields several pygidia closely similar to those referred to H. clarkei by Kozłowski (Reference Kozłowski1923) and Braniša (Reference Braniša1965). These pygidia show continuous morphologic variation toward typical pygidia of B. notica, as illustrated in Figure 6 herein and by Braniša (Reference Braniša1965, pl. 11, fig. 3, without taxonomic assignment). On the basis of this evidence, we consider that the pygidia originally assigned by Kozłowski (Reference Kozłowski1923) to the cephala of H. clarkei might actually belong to B. notica. This opinion coincides with that of Cooper (Reference Cooper1982, p. 39), who also proposed synonymy of B. notica and H. clarkei on the basis of pygidial characters. In contrast, Sandford (Reference Sandford2005, p. 23), in a detailed revision based on both cephalic and pygidial characters, considered the species clarkei to be incertae sedis at the generic level.
Clarification of the affinities of H. clarkei must await additional collections from the type area in Bolivia to establish the correct association of cephala and pygidia. An improved understanding of B. clarkei is also necessary to unravel the synonymy of several other species recognized in the Devonian of Uruguay by Méndez-Alzola (Reference Méndez-Alzola1938). For example, Sandford (Reference Sandford2005, p. 23) suggested that Homalonotus buqueti Méndez-Alzola, Reference Méndez-Alzola1938, and H. spatulirostris Méndez-Alzola, Reference Méndez-Alzola1938 are junior subjective synonyms of B. clarkei, whereas H. caorsi Méndez-Alzola, Reference Méndez-Alzola1938 is probably conspecific with B. notica.
Furthermore, Méndez-Alzola (Reference Méndez-Alzola1938) proposed new species in open nomenclature from Uruguay based on poorly preserved thoracic fragments that might be assigned to B. notica. These taxa include Homalonotus sp. alfa (Méndez-Alzola, Reference Méndez-Alzola1938, p. 59, pl. 12, fig. 7) and Homalonotus sp. beta (Méndez-Alzola, Reference Méndez-Alzola1938, p. 60, pl. 12 fig. 8).
Discussion
External exoskeletal characters used in taxonomy and their taphonomic origin.—The ornamentation of the external surface of B. herschelii and B. notica has often been described as granulose (Reed, Reference Reed1925, p. 185; Cooper, Reference Cooper1982, p. 20; Simões et al., Reference Simões, Leme and Soares2009, p. 30). However, the presence of granules was considered a preservational character (Cooper, Reference Cooper1982, p. 36; Simões et al., Reference Simões, Leme and Soares2009). Following this criterion, Cooper (Reference Cooper1982) interpreted the variety “africana” of Reed (Reference Reed1925) as a junior synonym of B. notica.
The origin of such a granulation can be interpreted from the microstructure of the carapace of Homalonotus rhinotropis Angelin, Reference Angelin1854 described by Dalingwater et al. (Reference Dalingwater, Hutchinson, Mutvei and Siveter1996, Reference Dalingwater, Siveter and Mutvei1999), from the Silurian of Sweden. They identified a thin “laminated outer layer = LOL” (Dalingwater et al., Reference Dalingwater, Siveter and Mutvei1999, fig. 1.1, 1.2), which is no more than 10% of the carapace thickness. In turn, an underlying “main layer” comprises most of the exoskeletal thickness (nearly 90%). These studies show abundant pore canals crossing the main layer from the internal side and emerging at the external surface (Dalingwater et al., Reference Dalingwater, Siveter and Mutvei1999, fig. 1.1, 1.2). Pores on the surface of the carapace are the external openings of the pore canals.
The “granules” are a distinctive feature among homalonotids, reflecting the microstructure of the carapace, and are conspicuous when the most internal layer is still present (i.e., when the decortication is not complete). They are a preservational artifact caused by the removal of the laminated outer layer (decortication), leaving the secondary filling of the pore canals exposed. The laminated outer layer is rarely preserved, although its absence is difficult to recognize because it is extremely thin.
A fragmentary cranidium of B. notica (CEGH-UNC 24.244) from the Cordón del Peñón section, in San Juan Province (Fig. 7.10, 7.11, 7.13, 7.14) exhibits exceptionally well-preserved areas of the carapace. Small pores vary in diameter between 0.035 mm and 0.2 mm, and are distributed in two distinct size classes, as described by Dalingwater et al. (Reference Dalingwater, Hutchinson, Mutvei and Siveter1996, Reference Dalingwater, Siveter and Mutvei1999), although in our case they are of a larger size. Pores tend to be less abundant and sparsely distributed to nearly absent on the highest and lowest areas of the carapace, such as the top of the lobes and the bottom of some furrows (e.g., LO). In the dorsal part of the glabella, pores are separated rather regularly, with a density of nearly 10 pores per mm2 (only 20% correspond to the larger size set), while towards the post-ocular area of the fixigena density is similar, but pores are larger and broadly similar in size. In some areas, however, the carapace of this specimen is partially decorticated. In these decorticated areas, the internal layers exhibit conical or mamelonar prominences, that may become columnar. These “granules” vary in diameter between 0.08 mm and 0.3 mm, and correspond in position with the pores of the external surface (Fig. 7.11, 7.13).
Additional Burmeisteria specimens from the concretionary level of the La Chilca section record complete elimination (internal molds), partial decortication, and well-preserved areas of the carapace in the same individual. When the outer layer is missing, the “granules” appear exclusively restricted to the internal layer (Figs. 3–6). An analogous pattern is clear in specimens of B. herschelii from the Falklands/Malvinas Islands illustrated by Carvalho (Reference Carvalho2006, pl. 1, figs. 3, 5, 6).
Hence, according to this evidence, the external surfaces of the carapaces of B. herschelii and B. notica are porous (instead of granulose). This conclusion largely agrees with observations by Eldredge (Reference Eldredge1970) on the microstructure of the carapace of Dipleura dekayi from the Middle Devonian of New York, recently corroborated by Carvalho (Reference Carvalho2018) in specimens from the Devonian Floresta Formation from Colombia. Interestingly, Carvalho (Reference Carvalho2018) pointed out that these pore canal fillings were previously interpreted as “tubulipore” epibionts (Hall and Clarke, Reference Hall and Clarke1888; Stumm, Reference Stumm1953).
Deeply decorticated specimens exhibiting a nearly smooth surface indicate that the internal layers are completely lost (i.e., they are true internal molds). Specimens of B. notica from the Middle Devonian of the Pimenteira Formation from Brazil, reported by Leme et al. (Reference Leme, Meira, Stasi and Soares2013), may record this condition.
It is worth noting that pore canals were interpreted as ducts for innervation of former sensory setae. Their function is controversial and might have varied according to their position in the exoskeleton, but the pores are most likely to be related to mechanoreception or chemoreception (e.g., Miller, Reference Miller1975; Haas, Reference Haas1981; Fortey and Owens, Reference Fortey, Owens and Savazzi1999a, among others). Clarkson (Reference Clarkson1975) and Horváth et al. (Reference Horváth, Clarkson and Pix1997) called attention to the presence of pits surrounding the eyes that would correspond to a sensory peripheral zone. The presence of two distinct size classes of pores in specimens of B. notica may indicate that more than one function was involved.
Several authors suggested a semi-infaunal lifestyle for homalonotid trilobites (Sdzuy, Reference Sdzuy1957; Hammann, Reference Hammann1985; Whittington, Reference Whittington1993), along with a predatory feeding habit (Fortey and Owens, Reference Fortey and Owens1999b). In this context, sensory receptors might have contributed by monitoring the environment, especially when the organism was in quiescent or resting infaunal or semi-infaunal position (Eldredge, Reference Eldredge1970, fig. 4 B, 4 C).
The ventral process of the rostral plate.—Burmeisteria has a well-developed triangular rostral plate, with the anterior rim and suture typically visible in dorsal view. This plate exhibits a ventro-anteriorly directed, stout spinose structure with a bulbose base, whose tip protrudes beyond the anterior cephalic margin (Figs. 3.1–3.6, 5.5, 5.8; Cooper, Reference Cooper1982, figs. 9, 10, 14, 28). The specimen CORD-PZ 8592 (Fig. 3.1–3.6) provides a good example of this structure. In addition, it is recognized in the original specimen of B. herschelii reported by Salter (Reference Salter1856, pl. 24, fig. 2) and re-illustrated by Cooper (Reference Cooper1982, fig. 10), and in the specimens of B. notica from the Devonian of the Paraná Basin from Brazil, illustrated by Clarke (Reference Clarke1913, pl. 1, pl. 2, figs. 3–6, pl. 3, fig. 3). This spinose projection appears to be homologous to that described by Wenndorf (Reference Wenndorf1990) for Digonus and Burmeisterella. He interpreted it as a coaptative structure, fitting into the upturned inner edge of the posterior pygidial doublure during complete enrollment. We propose that it might have functioned as a relatively efficient interlocking device, preventing release through a simple movement outwards in the sagittal plane. To unlock the pygidium from the head after enrollment, a combined movement of the rostral structure towards the interior of the pygidial cavity and to its anterior part would have been required. In fact, the pygidium and the cephalon would fit properly during enrollment only if this spinose structure docked with the posterior pygidial doublure. An interesting implication of this morfo-functional interpretation is that it would help the individual to remain enrolled for long periods with a minimum of muscular energy. Such adaptation would be particularly useful during persistent threat of vagrant predators, particularly fishes or cephalopods. This mechanical advantage might represent an additional strategy related to other passive (morphological) and active (behavioral) defensive traits previously suggested for these homalonotids, such as spinosity and infaunalism. Although there are no reports on direct predation in these trilobites, we document a malformation in a pygidium of B. notica (Fig. 7.7), which might be interpreted as damage scars resulting from a non-lethal attack. Both features, the more efficient interlocking device to protect soft parts and the possible evidence of a non-lethal predator attack, are significant in the context of the rising predation pressure proposed for mid Paleozoic times (see Klug, et al., Reference Klug, Kröger, Kiessling, Mullins, Servais, Frýda, Korn and Turner2010 and references therein) and its evolutionary consequences on trilobites (see Rustán et al., Reference Rustán, Balseiro, Waisfeld, Foglia and Vaccari2011 and references therein).
Stratigraphic and geographic distribution
As outlined above, the taxonomy of Digonus and Burmeisteria has been discussed extensively. Digonus was reported from the high latitudinal southwestern Gondwanan basins (the Malvinokaffric Realm) either as a genus or as a subgenus of Burmeisteria. However, as revised by Sandford (Reference Sandford2005), Digonus no longer occurs in the southwestern (Malvinokaffric) Gondwanan basins. In contrast, Burmeisteria has a distinct endemic Malvinokaffric distribution, restricted to South Africa, Ghana, the Falklands/Malvinas Islands, southern South America, and Antarctica. However, a putative record from the Lower Devonian of Algeria has been cited by Simões et al. (Reference Simões, Leme and Soares2009, p. 38, table 3).
Species of Burmeisteria that attain the widest distribution are B. herschelii and B. notica, with records in the Andean, Brazilian, and South African provinces of the Malvinokaffric Realm (according to provincial divisions proposed by Eldredge and Ormiston, Reference Eldredge, Ormiston, Gray and Boucot1979). In Argentina, these two species represent the only Devonian homalonotids reported to date. South Africa adds B. fontinalis (Reed, Reference Reed1925), but in the Fox Bay Formation from the Falklands/Malvinas Islands, the only homalonotid recorded is B. herschelii. In contrast, Brazilian (cratonic) basins yield not only B. oiara and B. clarkei, but also Burmeisterella (Carvalho, Reference Carvalho2005), a taxon also recorded outside the Malvinokaffric Realm. Bolivian (Andean) basins exhibit a similar diversity of Burmeisteria species to the cratonic region (but see above taxonomic remarks about the species reported by Pek and Vaněk, Reference Pek and Vaněk1991). In addition, Bolivian strata record the Lower–Middle Devonian Dipleura, a nearly cosmopolitan genus with extensive records from the Hamilton Group of the USA, but also present in Colombia, France, Poland, Spain, and Germany (Sandford, Reference Sandford2005; Carvalho, Reference Carvalho2018).
The biostratigraphic range of Burmeisteria is late Silurian (Ludlow) to Middle Devonian (Givetian?) according to the records from the Silurian Catavi and El Carmen formations of Bolivia (Edgecombe and Fortey, Reference Edgecombe and Fortey2000) and the mainly Middle Devonian Pimenteira Formation of Brazil (Simões et al., Reference Simões, Leme and Soares2009; Leme et al., Reference Leme, Meira, Stasi and Soares2013). Thus, the Early Devonian should be considered the acme of Burmeisteria because this interval records the highest number of species.
In the Argentine Precordillera, Burmeisteria is almost exclusively restricted to an interval of Pragian bluish-gray, fine-grained wackestone in the middle part of the Talacasto Formation (Fig. 2). It is extremely scarce in the lowermost dark muddy interval of the unit and, so far, its uppermost occurrence is in the top of the Keidel's marker horizon (Pragian). Abundance of Burmeisteria in the Precordillera basin is highest in shallow-water depositional facies (Astini, Reference Astini1991). These occurrences match previous observations on environmental preferences of homalonotids, from different ages and units, mostly from siliciclastic platform settings (Gill, Reference Gill1948; Thomas, Reference Thomas1979; Chlupáč, Reference Chlupáč1981; Fortey and Owens, Reference Fortey, Owens and Kaesler1997; Mikulic and Kluessendorf, Reference Mikulic and Kluessendorf1999; Sandford, Reference Sandford2005; Crônier and Van Viersen, Reference Crônier and Van Viersen2007; Simões et al., Reference Simões, Leme and Soares2009). In particular, these trilobites are most frequent in depths between the fair-weather and the storm wave-base levels, and such an ecological restriction hinders their use in biostratigraphy at regional or larger scales. However, Burmeisteria (a locally abundant taxon, of relatively large size, and easily recognizable) helps to correlate Pragian strata underlying the Keidel's marker horizon, in the Precordillera basin.
Acknowledgments
The authors acknowledge support from the National Research Council of Argentina (CONICET), PUE granted to CICTERRA, from University of La Rioja project 2017-6993 granted to J. J. Rustán and E. Vaccari; and from the Agencia Nacional de Promoción Científica y Tecnológica PICT 2017-3095 granted to J. J. Rustán.
The Compañía Minera del Pacífico and its local manager, engineer E. Alonso, provided permission to access the outcrops in Loma de los Piojos section. Thanks are extended to the reviewers and the editorial staff for their helpful comments and suggestions that significantly improved the final version of the manuscript. G. McLellan improved the English version of the manuscript.
Accessibility of supplemental data
Data available from the Dryad Digital Repository: DOI https://doi.org/10.5061/dryad.vt4b8gtn8.









