Introduction
In late 2011, the Australian research vessel Southern Surveyor undertook dredging operations on a number of bathymetric highs (Batavia Knoll, Gulden Draak Knoll, and Dirk Hartog Ridge; Fig. 1) on the eastern Indian Ocean seafloor as part of long-term research led by the University of Tasmania and University of Sydney. The primary objective was to ascertain the nature of the knolls and obtain a greater understanding of the tectonic history of the Australia–India–Antarctica divergence, for which the Perth Abyssal Plain is the focus. As a result of these studies, Williams et al. (Reference Williams, Whittaker, Granot and Müller2013) determined the knolls were microcontinents, fragments of Greater India isolated during the separation of that plate from the Australian Plate. The steep northwestern scarp of Batavia Knoll was dredged (between –25.332º, 100.285º and –25.343º, 100.298º and depths of 2,870 to 2,730 m; Whittaker et al., Reference Whittaker, Halpin, Williams, Hall, Gardner, Kobler, Daczko and Müller2013), and rock samples of sandstone were recovered therefrom, which proved to be fossiliferous and yielded the first paleontological suite from this region. This collection includes diverse assemblages of macroinvertebrates (Mollusca: Cephalopoda, Bivalvia, Gastropoda, Scaphopoda; Annelida; probable Echinodermata fragments), microinvertebrates (Ostracoda; Foraminifera), and terrestrially derived palynomorphs of spores and pollen. Substantial segments of pholadoid-bored wood are also recorded, attesting to shallow habitats proximal to the shoreline. This paper documents the taxonomic composition and paleoecological significance of the mollusks, annelids, and other invertebrate fossils recovered from Batavia Knoll.

Figure 1 (1) Simplified bathymetric map showing features in and around the Perth Abyssal Plain (on left) and a reconstruction of the PAP region ca. 100 Ma (on right), with a fixed Australian reference frame (adapted from Gibbons et al., Reference Gibbons, Barckhausen, van den Bogaard, Hoernle, Werner, Whittaker and Müller2012). Black diamond marks the location of dredging; thin black line across knoll delineates profile; thick black line represents active spreading ridges; dotted black line indicates the extinct spreading center of the PAP. (2) East–west profile of Batavia Knoll, 5x vertical exaggeration. Aus=Australia; Ant=Antarctica; B=Batavia Knoll; DHR=Dirk Hartog Ridge; GD=Gulden Draak Knoll; Ind=India; NP=Naturaliste Plateau; PAP=Perth Abyssal Plain; WB=Wharton Basin.
Repositories and institutional abbreviations
Specimens described herein are housed at the Western Australian Museum (WAM), Perth, and are cataloged with the call number 15.1XX. Other institutions referenced in the text are the Sedgwick Museum (SM), Cambridge; the Geological Society of London Museum (GSL) and the Natural History Museum (NHM), London; and the Geological Survey of India Museum (GSI), Kolkata.
Geological and depositional setting
Batavia Knoll sits on the western limit of the Perth Abyssal Plain, approximately 1,600 km west of the Western Australian coast, the top at a depth of between 1,700 and 2,000 m below sea level (Fig. 1.2). Sedimentary samples recovered therefrom consist of a mixture of sandstone (medium-grained laminated to coarse-grained massive) facies. Those studied herein exhibited low textural maturity, being poorly sorted, having relatively large subhedral quartz (which dominated; ~80% framework composition), and smaller mica and feldspar framework grains supported by a micritic matrix. The sandstone is massive, and all fossils therein were arranged with haphazard orientations, with no bedding apparent. These fossils are primarily preserved as molds, indicating the rock was permeated by fluid, resulting in the dissolution of original, predominantly aragonitic, shell material through geologic time. Because all rocks were obtained from dredging operations, the relationship between the studied sample and other recovered lithological samples is unknown. Seismological surveys have not yet been conducted across the knoll to ascertain the subsurface structure of the rock units, though the presence of a few small half-graben basins has been inferred (Whittaker et al., Reference Whittaker, Halpin, Williams, Hall, Gardner, Kobler, Daczko and Müller2013). Given the half-graben’s similar size and orientation to basins on the Naturaliste Plateau, which is conjugate with southern Batavia Knoll, it is likely these depocenters were formed during the divergence of Australia–Antarctica from Greater India, a process that commenced ca. 132 Ma (Gibbons et al., Reference Gibbons, Barckhausen, van den Bogaard, Hoernle, Werner, Whittaker and Müller2012). A westward ridge jump of the spreading center between ca. 108 and 103 Ma resulted in the rifting of Batavia Knoll and Gulden Draak Knoll, further to the south, from Greater India and the opening of the Wharton Basin (Williams et al., Reference Williams, Whittaker, Granot and Müller2013).
We surmise the separation of Batavia Knoll from its parent continent opened a narrow inlet between the two continental blocks (at least one of which, and likely both, was subaerial), with restricted access to global oceanic currents. This supposition is founded on the level of endemism of Mollusca in the Batavia Knoll specimens, indicating poor dispersal capabilities of the marine fauna there. At least 22% of recovered mollusks (at species level) are newly described herein, a relatively high amount considering Batavia Knoll falls within a paleobiogeographic region whose fauna have been robustly studied since the 1840s (e.g., Forbes, Reference Forbes1846). Endemism here is also consistent with known patterns of provincialization resulting from East Gondwana breakup, though these tectonic and paleobiogeographic invertebrate patterns will be explored in a future article and are beyond the scope of this paper. It is into this embayment that the sandstones recovered from Batavia Knoll were deposited.
The poorly sorted, polymictic composition of the samples, together with the lack of sedimentary structures, indicates these rocks were formed during a mass-flow event, which propagated a short distance across the continental shelf before terminating in a shallow marine environment. Close proximity of the deposition site to the paleoshore is evidenced in the presence of a substantial quantity of wood fragments being recovered from the Batavia Knoll sandstone. The majority of these lignitic ‘clasts’ were heavily bored by pholadoid bivalves (assigned to ichnospecies Teredolites longissimus Kelly and Bromley, Reference Kelly and Bromley1984, and associated with the families Teredinidae Rafinesque, Reference Rafinesque1815 and Pholadidae Lamarck, Reference Lamarck1809; see Fig. 4.14 in Systematic paleontology).
Age determination
The majority of macroinvertebrate taxa recovered from Batavia Knoll belong to long-ranging families, which are present throughout the Mesozoic and Cenozoic. The only families that do not exhibit this expansive range are those of the ammonites, which appeared in the Early Cretaceous—Desmoceratidae (which first appeared in the Valanginian) and Hamitidae (which arose during the Albian; Wright et al., Reference Wright, Calloman and Howarth1996)—thus delimiting the maximum age for the associated strata to the Albian (Fig. 2). Numerous other taxa first appeared in the Albian, including the genera Drepanocheilus Meek, Reference Meek1864, Igonoia Squires, Reference Squires2011, and Bhimaites Matsumoto, Reference Matsumoto1954, which are recorded in Batavia Knoll deposits. Nuculana socialis Stoliczka, Reference Stoliczka1871 is only known from Albian to Cenomanian strata (Stoliczka, Reference Stoliczka1871; Berizzi and Busson, Reference Berizzi and Busson1971). Furthermore, in his original description of the species, d’Orbigny (Reference d’Orbigny1843, p. 340) states that Panopea inaequivalvis is prevalent in, and characteristic of, Albian strata of France.

Figure 2 Taxonomic list of all taxa recovered from Batavia Knoll, showing mid-Cretaceous age ranges for each taxon and delimiting the age of the unit to the latest Albian. Asterisks indicate ranges established in this study. Apt.=Aptian; Turo.=Turonian. Absolute ages from Gradstein and Ogg (Reference Gradstein and Ogg2012).
Aucellina gryphaeoides Sowerby, Reference Sowerby1836 is not recorded below the base of the Mortoniceras inflatum Ammonite Zone in Europe (Lehmann et al., Reference Lehmann, Tröger and Owen2008), and Bhimaites stoliczkai Kossmat, Reference Kossmat1898 is found to have a similarly timed first appearance datum there (Gale et al., Reference Gale, Brown, Caron, Crampton, Crowhurst, Kennedy, Petrizzo and Wray2011). Huang et al. (Reference Huang, Ogg and Hinnov2012) dated the base of the M. inflatum zone to 103.94 Ma. The type specimen for B. stoliczkai was described from the Odiyam Member of the Karai Formation in southern India (Stoliczka, 18Reference Stoliczka65), a unit that straddles the Albian-Cenomanian boundary (Sundaram et al., Reference Sundaram, Henderson, Ayyasami and Stilwell2001). The temporal range of both these taxa extends only into the early Cenomanian (Morter and Wood, Reference Morter and Wood1983; Kennedy and Klinger, Reference Kennedy and Klinger2014), restricting the possible age to an approximately 6 Myr window. Further refinement comes with the extinction of Planohamites Monks, Reference Monks2002 at the end of the Albian, 100.5 Ma (Monks, Reference Monks2002; Gradstein et al., 2012).
The presence of these temporally restricted taxa resolves the age of the Batavia Knoll sandstone, constraining it to the late Albian and probably the early late Albian, ca. 103 to 101 Ma. This age estimation falls within the period of extension between Batavia Knoll and Greater India calculated by Williams et al. (Reference Williams, Whittaker, Granot and Müller2013). Given the sample site’s location on the northwest edge of the knoll, an area conjugate with Greater India (and thus prior to separation a likely terrestrial environment), the genesis of this divergence allowed for the creation of marine conditions in the area, in which the Batavia Knoll fauna could inhabit shallow waters.
An Albian age of the Batavia Knoll sandstone is also supported by the microfossil taxa present. The two ostracod taxa recovered belong to the Trachyleberididae, an extant family with origins in the late Barremian (Karpuk and Tesakova, Reference Karpuk and Tesakova2014) and thus present throughout the Albian. In addition, the genus Spinoleberis Deroo, Reference Deroo1966 has its first appearance datum in the mid-Albian. Foraminifera recovered are consistent with assemblages recorded by Scheibnerová (Reference Scheibnerová1972) during Leg 27 of the Deep Sea Drilling Program in the eastern Indian Ocean, including the Perth Abyssal Plain. The benthic foraminiferal assemblages therefrom were deemed to be of late Albian age. This is consistent with preliminary calcareous microfossil analysis conducted by P. Quilty (personal communication, 2014). Reliable palynological data recovered from Batavia Knoll are extremely sparse (three spore-pollen grains only); investigation of it did not contradict a mid-Cretaceous age, but offered no further refinement.
Paleoecology
The fossil suite of Batavia Knoll encompasses four primary autecological groups: suspension feeders (both infaunal and epifaunal), grazers (epifaunal), deposit feeders (infaunal), and carnivores (epifaunal and nektic). These data are summarized in Figure 3. Gastropod taxa account for 42% of the specific composition, and bivalves 31%. Cephalopoda and Ostracoda each account for 8% (with two representative taxa recovered), and Scaphopoda, Polychaeta, and probably Echinoidea, with one taxon apiece, make up 4% each of the rock’s assemblage.

Figure 3 Pie and bar charts of the relative abundance of autecological groups within the Batavia Knoll fossil suite, excluding the recovered Ostracoda and probable echinoid. (1) Life habits. (2) Feeding mode. EB=epifaunal byssate; EM=epifaunal mobile; DI=deep infaunal; IB=semi-infaunal byssate; SI=shallow infaunal.
Suspension feeders account for 39% of the biota of the suite, represented by 10 genera (Turritella, Parsimonia, and all bivalves except Nuculana). Of these, the majority is infaunal, with only the serpulid and Aucellina occupying epifaunal niches. Most of the filter feeders obtained their nutrients singularly from suspension, though some potentially adopted a bimodal feeding habit. Modern turritellids are predominantly suspension feeders, but some species exhibit both detritivorous and grazing habits (Allmon, Reference Allmon1988; Allmon et al., Reference Allmon, Jones and Vaughan1992). They are here counted among the suspension feeders only.
Nuculanoid bivalves are known to obtain nutrients by sediment consumption (Bender and Davis, Reference Bender and Davis1984), as are aporrhaid gastropods (Barnes and Bagenal, Reference Barnes and Bagenal1952). According to Houbrick (Reference Houbrick1992) modern cerithiids graze on diatoms and microalgae, though his review of Argyropeza Melvill and Standen, Reference Melvill and Standen1901, the only extant genus of the Procerithiidae, indicates they are deposit feeders (Houbrick, Reference Houbrick1980). Which characteristic is ascribable to fossil taxa is unknown; the cerithioid taxa herein (Procerithium and Cirsocerithium) are considered among the deposit feeders, totaling five species (22%) utilizing this feeding mode. The herbivorous, motile grazers are represented by the margaritid and calliotropid taxa.
Carnivores comprise the remainder of the recovered population (30%), represented by a pleurotomariid, two members of the Architectonicidae, a ringiculid, two ammonite taxa, and a single dentaliid species. The pleurotomariid and architectonicid gastropods were epifaunal, the dentaliid shallowly infaunal, and the two ammonites were highly active, nektonic predators.
The presence of bored wood fragments in the fossil record of Batavia Knoll is indicative of infestation by xylophagous bivalves, which inhabit and consume driftwood and other lignitic substrates (Sellius, Reference Sellius1733). Pholadoid bivalves can consume at least 60 cm3 in wood material per year per 100 individuals (Amon et al., Reference Amon, Sykes, Ahmed, Copley, Kemp, Tyler, Young and Glover2015), suggesting that such substantial fragments of wood would not survive an extended period in marine conditions. Thus, due to their presence, the depositional site is likely in proximity to vegetated land. These autecological and paleoecological data strengthen the sedimentological interpretation of the depositional site as a shallow marine environment. The ratio of carnivorous, detritivorous, and suspension-feeding taxa in the Batavia Knoll suite plot directly in the inner shelf environment in the trophic analysis model of Scott (Reference Scott1978). Further, the taxonomic composition and trophic structure of Batavia Knoll invertebrates can be estimated for the first time, providing insights into shallow marine Cretaceous habitats in a previously unstudied area of the planet.
Systematic paleontology
The majority of taxa are described from casts, taken using Pinkysill® Putty, a two-part silicone press putty. Bivalve taxonomic hierarchy follows Carter et al. (Reference Carter2011), and that of the gastropods follows Bouchet and Rocroi (Reference Bouchet and Rocroi2005), with addenda of relevant taxa from works post–2005.
Phylum Mollusca Linnaeus, Reference Linnaeus1758
Class Bivalvia Linnaeus, Reference Linnaeus1758
Subclass Protobranchia Pelseneer, Reference Pelseneer1889
Order Nuculanida Carter, Campbell, and Campbell, Reference Carter, Campbell and Campbell2000
Superfamily Nuculanoidea Adams and Adams, Reference Adams and Adams1858 (1854)
Family Nuculanidae Adams and Adams, Reference Adams and Adams1858 (1854)
Genus Nuculana Link, Reference Link1807
Type species
Arca rostrata Bruguière, Reference Bruguière1789 (=Arca pernula Müller, Reference Müller1779) by original designation.
Nuculana sp. cf. N. socialis Stoliczka, Reference Stoliczka1871
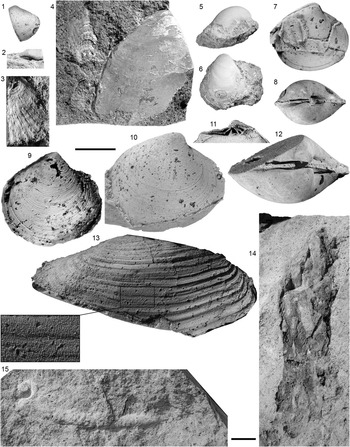
Figure 4 Cretaceous Bivalvia recovered from Batavia Knoll. (1, 2) Nuculana sp. cf. N. socialis Stoliczka, Reference Stoliczka1871, WAM 15.120: (1) cast of right valve; (2) dorsal view of steinkern exhibiting taxodont dentition. (3) Spondylus sp., left valve, WAM 15.160. (4) Pinnidae gen. indet. sp. indet, WAM 15.106 (on left) and Inoceramus sp., WAM 15.105 (on right). (5, 6) Aucellina gryphaeoides Sowerby, Reference Sowerby1836, left valves: (5) WAM 15.148; (6) WAM 15.150. (7, 8) Resatrix sp., WAM 15.130: (7) cast of right valve; (8) steinkern. (9) Astarte jugosa Forbes, Reference Forbes1846, cast of right valve, WAM 15.135. (10–12) Resatrix sp.: (10) cast of right valve, WAM 15.139; (11) cast of right valve detailing hinge dentition, WAM 15.126; (12) steinkern, WAM 15.104. (13) Panopea sp. cf. P. inaequivalvis d’Orbigny, Reference d’Orbigny1842, cast of right valve, with inset showing shagreen texture (height of box 5 mm), WAM 15.137. (14) Oxidized wood fragment showing pholadoid Teredolites longissimus Kelly and Bromley, Reference Kelly and Bromley1984 bore traces, WAM 15.153. (15) Probable venerid bivalve escape burrow traces, WAM 15.103. (1–13) Specimens coated with ammonium chloride. Scale bar=1 cm; (1–13) use bar above 9, 10; (14, 15) use bar between them.
1871 cf. Nuculana socialis Stoliczka, p. 323, pl. 17, fig. 13.
1971 cf. Nuculana (sensu stricto) socialis; Berizzi and Busson, p. 468, pl. 35, fig. 4.
Occurrence
Northern Africa and southern India; Albian to Cenomanian.
Description
Comparatively small shell for genus, but large for species (reconstructed length ~15 mm). Outline subovate; from beak anterior margin moderately declivous, until confluence with moderately convex ventral margin. Dorsoposterior margin not preserved. Inflation moderate. Ornamentation of at least 34 evenly spaced commarginal growth lines, which become more distinct toward the ventral margin. Umbonal region is almost smooth. Taxodont dentition comprises at least 10 small peg-like teeth along the anterodorsal margin.
Materials
Single right valve mold and steinkern.
Remarks
The length and posterior features of the specimen are unknown as the single recovered valve was inadvertently dissected by a rock saw during preparation of the rock. Whether it exhibits the cuneiform posterior habit of N. socialis is unclear and thus is left in open nomenclature. If this specimen is conspecific with N. socialis, it represents a very large individual of that species as its type material is a third of the size of this specimen (length 4.5 mm, height 2.9 mm; Kendrick and Vartak, Reference Kendrick and Vartak2007). Stoliczka (Reference Stoliczka1871) notes it is a very common species around ‘Moraviatoor’ (now Maruvattur) in the Dalmiapurum Formation, Uttattur Group (late Albian; Sundaram et al., Reference Sundaram, Henderson, Ayyasami and Stilwell2001) of southern India. Berizzi and Busson (Reference Berizzi and Busson1971) record N. (s.s.) socialis in Cenomanian strata of Djebel Zmertene, Tunisia.
Subclass Autobranchia Grobben, Reference Grobben1894
Infraclass Pteriomorphia Beurlen, Reference Beurlen1944
Order Myalinida Paul, Reference Paul1939
Superfamily Inoceramoidea Giebel, Reference Giebel1852
Family Inoceramidae Giebel, Reference Giebel1852
Genus Inoceramus Sowerby, Reference Sowerby1814
Type species
Inoceramus cuvierii Sowerby, Reference Sowerby1814, by subsequent designation (Cox, Reference Cox1955).
Inoceramus sp.
Description
Shell small for genus, preserved length 22.8 mm. Anterior margin well rounded. Moderately inflated. Ornamentation of broad commarginal symmetrical rugae forming corrugations over entirety of shell, with two or three finer commarginal striations present in the interstacies. Poorly developed radial sculpture present, approximately two striae per millimeter across whole of shell disc, more distinct dorsally.
Materials
One decorticated left valve fragment.
Remarks
The shell margins of the recovered specimen are not preserved. Notwithstanding this, the preserved characteristics of the shell conform to those of a relatively small specimen of Inoceramus. It could be conferrable to I. geinitzianus Stoliczka, Reference Stoliczka1871 of southern India due to its dimensions (though that species can obtain lengths of 160 mm), degree of inflation, and broad rugose texture with finer interstitial threads (which Stoliczka, Reference Stoliczka1871, p. 407 states are compact and tightly spaced, a feature that may be obscured by the decorticated nature of the Batavia Knoll specimen). The fine radial sculpture, while not discussed by Stoliczka (Reference Stoliczka1871) in his original description, is depicted in his figures of the species (pl. 27, fig. 5a). Inoceramus geinitzianus has been recorded from late Albian to late Turonian rocks in India (Stoliczka, Reference Stoliczka1871; Kendrick and Vartak, Reference Kendrick and Vartak2007).
Order Pterioidea Gray, Reference Gray1847 (Goldfuss, Reference Goldfuss1820)
Superfamily Pinnoidea Leach, Reference Leach1819
Family Pinnidae Leach, Reference Leach1819
Pinnidae gen. indet. sp. indet.
Description
Shell subtriangular in outline, relatively small for family (maximum measurable height 13.8 mm). Hinge and ventral margins are unknown, apical angle ~60º. Ornamentation of fine radial riblets, crossed by broad, widely spaced commarginal terraces.
Materials
Two fragments preserving original shell material.
Remarks
These specimens exhibit the characteristic trigonal wedge shape of Pinnidae but are quite short for the family. Both recovered specimens are fragmentary single valves.
Order Pectinida Gray, Reference Gray1854
Superfamily Pectinoidea Rafinesque, Reference Rafinesque1815
Family Spondylidae Gray, Reference Gray1826
Genus Spondylus Linnaeus, Reference Linnaeus1758
Type species
Spondylus gaederopus Linnaeus, Reference Linnaeus1758, by subsequent designation (Schmidt, Reference Schmidt1818).
Spondylus sp.
Description
Shell small for genus. Outline obliquely broadly ovate to subtrigonal. Anterior margin straight to merge with highly convex ventral margin. Umbo rounded, raised, delineated by angular ridge, which abruptly descends to shell margin. Ornament of evenly spaced coarse radial ribs, intermittently nodulate, with interstices approximately four times the width of the rib. The coarse ribs sometimes extend beyond the ventral margin of shell. Within the interstices three to five fine radial riblets are present. Slight concentric undulations result in a fine commarginal rugose texture.
Materials
Two broken decorticated original shell fragments.
Remarks
The ornament of these specimens is most closely akin to that of Spondylus calcaratus Forbes, Reference Forbes1846 (p. 155, pl. 18, fig. 2) from the Turonian to Santonian of southern India, though these specimens are much smaller than any recorded S. calcaratus and are not contemporaneous.
Superfamily Buchioidea Cox, Reference Cox1953 (Fischer, Reference Fischer1886)
Family Buchiidae Cox, Reference Cox1953 (Fischer, Reference Fischer1886)
Genus Aucellina Pompeckj, Reference Pompeckj1901
Type species
Avicula gryphaeoides Sowerby, Reference Sowerby1836 (non Sedgwick, Reference Sedgwick1829), by subsequent designation (Marwick, Reference Marwick1939).
Aucellina gryphaeoides Sowerby, Reference Sowerby1836
1836 Avicula gryphaeoides Sowerby, p. 335, pl. 11, fig. 3.
1893 Avicula gryphaeoides; von Reference von StrombeckStrombeck, p. 490.
1901 Aucellina gryphaeoides; Pompeckj, p. 354, pl. 16, figs. 6–8.
1902 Aucellina gryphaeoides; Reference WollemannWollemann, p. 64, pl. 3, figs. 2, 3.
1905 Aucellina gryphaeoides; Woods, p. 72, pl. 10, figs. 6–13.
1936 Aucellina gryphaeoides; Reference RennieRennie, p. 315.
1958 Aucellina gryphaeoides; Reference von ZittelGlaessner, p. 203, pl. 24, fig. 1, text fig. 2.
Holotype
Lost. Neotype SM B21972 (Woods, Reference Woods1905, pl. 10, fig. 6), selected by Morter and Wood (Reference Morter and Wood1983); Cambridge Greensand, England; early Cenomanian.
Occurrence
Europe, northern Australia; Early Cretaceous (late Albian) to Late Cretaceous (mid-Cenomanian).
Description
Moderately small for genus, maximum recorded height ~20 mm. Strongly prosocline in outline, highly inequivalved. Left valve strongly convex with projecting umbo and prosogyous beak. Right valve almost flat and sitting entirely within left valve, being suborbicular in outline. Dorsal margins of right valve subhorizontal until merging with convex, well-rounded anterior and posterior margins. Ornamentation of left valve is of thin commarginal growth lirae, widely spaced near to beak (one to three per millimeter) becoming coarser and more concentrated toward ventral margin (about six per millimeter). Fine radial sculpture on umbo, becoming rapidly indistinct. Right valve similarly adorned with radial striae.
Materials
Numerous fragmentary original shell fragments, the majority of them preserving the beak, including two juvenile specimens, while some preserve the disc only.
Remarks
All specimens of this species were of original shell material, predominantly found clustered near the exterior of the sandstone boulder. The majority of specimens were broken, with only the beak region of the left valve preserved in most cases. Many of the unidentifiable shell fragments found in the same area of the boulder likely belong to Aucellina. The only right valve recovered was from a single minute juvenile specimen. The paucity of right valves is possibly due to their smaller size and relatively flat profile, making them easier to overlook when examining the rock, or the fact that many of the preserved left valves have not been completely prepared out of the rock. Two juveniles and a subadult specimen were also found. The umbo and byssal ear/notch of right valve are often obscured due to sediment impediment, and the specimens are too small and fragile to clean further.
Aucellina gryphaeoides is an index species for late Albian to mid-Cenomanian strata throughout Europe (Morter and Wood, Reference Morter and Wood1983).
Infraclass Heteroconchia Hertwig, Reference Hertwig1895
Order Carditida Dall, Reference Dall1889
Superfamily Crassatelloidea Férussac, Reference Férussac1822
Family Astartidae d’Orbigny, Reference d’Orbigny1844
Subfamily Astartinae d’Orbigny, Reference d’Orbigny1844
Genus Astarte Sowerby, Reference Sowerby1817
Type species
Venus scotica Maton and Racket, Reference Maton and Racket1807 by original designation.
Astarte jugosa Forbes, Reference Forbes1846
1846 Lucina jugosa, Forbes, p. 142, pl. 17, fig. 7.
1871 Grotriania jugosa; Stoliczka, p. 289, pl. 10, figs. 12–14.
Holotype
GSL R10593 (Forbes, Reference Forbes1846, pl. 17, fig. 7); ‘Pondicherry,’ southern India; Late Cretaceous. (Note: this specimen has been held by the NHM since 1911 [J. Todd, personal communication, 2016] but had not been formally registered within their collection at time of writing [March 2016], and thus does not yet have an official NHM number.)
Description
Shell average size for genus (25 mm long). Outline suborbicular, slightly longer than high. Umbo is prominent, angular, and protruding. Posterior margin suborthogonal from beak to junction with highly convex ventral margin. Anterior margin slightly convex until merging with ventral margin. Low inflation (laterally compressed), suggesting both lunule and escutcheon are narrow. Ornamentation of ~95 regularly spaced commarginal ribs, highly concentrated toward apex of umbo, strengthening ventrally.
Occurrence
India; Early Cretaceous (middle Albian) to Late Cretaceous (Cenomanian).
Materials
Single right valve.
Remarks
There is a great need to revise the descriptions and taxonomic positions of superficially orbicular bivalves from southern India, as the majority of work done in this area was completed in the nineteenth century and has not undergone significant revision since. The specimen recovered from Batavia Knoll could be reasonably assigned to several genera. The lack of a distinct anterior abductor scar on the preserved specimen, however, precludes it from being considered a member of the Lucinidae, and it is likely to be an astartid.
Stoliczka (Reference Stoliczka1871) recorded ‘Grotriania’ jugosa from limestone near Odiyam in the Uttatur Group. The only limestone in the vicinity of Odiyam is the Dalmiapuram Formation of late Albian age (Sundaram et al., Reference Sundaram, Henderson, Ayyasami and Stilwell2001). Forbes (Reference Forbes1846) only gives the locality of his specimens as ‘Pondicherry.’ The Pondicherry subbasin, within Cauvery Basin, is dated between late Campanian to Maastrichtian and into the Paleocene (Sundaram et al., Reference Sundaram, Henderson, Ayyasami and Stilwell2001). Astarte jugosa (as Grotriania) is noted as being in the Bagh Beds of central India (Chiplonkar, Reference Chiplonkar1939), which have dated to the Cenomanian (Chiplonkar, Reference Chiplonkar1941).
Order Cardiida Férussac, Reference Férussac1822
Superfamily Veneroidea Rafinesque, Reference Rafinesque1815
Family Veneridae Rafinesque, Reference Rafinesque1815
Subfamily Meretricinae Gray, Reference Gray1847 (Gray, Reference Gray1838)
Genus Resatrix Casey, Reference Casey1952
Type species
Resatrix dolabra Casey, Reference Casey1952 by original designation.
Resatrix sp.
1871 cf. Cytherea lassula Stoliczka, p. 171, pl. 7, figs. 10–17.
Description
Shell size typical for the genus; shell trigonally ovate to subcircular in outline, posterior and ventral margins convex, anterior margin subangular. Umbo prominent and projecting toward anterior. Inflation moderate. Escutcheon narrow, lunule shallow, almost absent. Ornamentation of at least 80 fine commarginal striations strengthening from almost smooth umbonal region to ventral margins. Heterodont dentition, right valve with narrow 3a and 1, strongly bifid 3b, anterior laterals AIII and AI elongate, posterior laterals absent. Dentition of left valve unknown.
Materials
Numerous articulated (or almost so) molds and steinkerns.
Remarks
This species is similar to bivalves described from the Ariyalur Group (Santonian to Maastrichtian; Sundaram et al., Reference Sundaram, Henderson, Ayyasami and Stilwell2001) in southern India by Stoliczka (Reference Stoliczka1871). He assigned them to Cytherea Lamarck, Reference Lamarck1806, a genus since invalidated as a junior homonym of a hexapod genus, and gave them the specific epithet lassula. Species within Cytherea were transferred into Meretrix Lamarck, Reference Lamarck1799, though the general form of Meretrix is not consistent with the Batavia Knoll samples. Tapaswi (Reference Tapaswi1987) assigned Stoliczka’s Cytherea species to Mesocallista Cox, Reference Cox1952. Dentition of Batavia Knoll specimens, however, places them within Resatrix, extending the temporal range of Resatrix from the early Albian (Casey, Reference Casey1961) to the late Albian and potentially into the Cenomanian.
Order Hiatellida Carter in Carter et al., Reference Carter2011
Superfamily Hiatelloidea Gray, Reference Gray1824
Family Hiatellidae Gray, Reference Gray1824
Genus Panopea Ménard de la Groye, Reference Ménard de la Groye1807
Type species
Mya glycimeris Gmelin, Reference Gmelin1791, by subsequent designation (Schmidt, Reference Schmidt1818).
Panopea sp. cf. P. inaequivalvis d’Orbigny, Reference d’Orbigny1843
1843 cf. Panopea inaequivalvis Reference d’Orbignyd’Orbigny, p. 340, pl. 358, figs. 5–7.
1949 cf. Panopea inaequivalvis; Reference CollignonCollignon, p. 25, pl. 4, fig. 1.
Occurrence
Madagascar and France; Early Cretaceous (Albian).
Description
Elongate to subquatrate shell, relatively small and narrow for genus. Posterior edge is elongated, with well-rounded, slightly gaping margins. Umbonal region is positioned about one-third of the way in from the obliquely truncated anterior margin. Moderate inflation. Ornamentation dominated by ~25 prominent, equally spaced commarginal ribs. These become broader and more widely spaced toward the ventral margin. Smaller commarginal striations present on the ridges and interstices of the larger better developed ribs. Entire surface of the shell textured with numerous, subequidistant, radial rows of minute tubercules.
Materials
Single slightly disarticulated mold with steinkern preserved.
Remarks
The identification of this specimen as Panopea inaequivalvis is uncertain due to the unusual microtuberculate texture found across the disc of the shell. Extensive texture of this nature is not a feature before observed in the Hiatellidae, and the only microtuberculate ornamentation recorded for the family is on the juvenile dissoconch of Hiatella (Pseudosaxicava) Chavan, Reference Chavan1952 from the mid–Jurassic of Europe (Schneider and Kaim, Reference Schneider and Kaim2011). Otherwise, this specimen conforms well to the characteristics of P. inaequivalvis, with the possible exception of not having quite so abrupt truncations of its anterior and posterior margins, as observed in some individuals of this species.
Class Gastropoda Cuvier, Reference Cuvier1795
Subclass Vetigastropoda von Salvini–Plawen, Reference von Salvini-Plawen1980
Superfamily Pleurotomarioidea Swainson, Reference Swainson1840
Family Pleurotomariidae Swainson, Reference Swainson1840
Genus Leptomaria Eudes-Deslongchamps, Reference Eudes-Deslongchamps1864
Type species
Pleurotomaria amoena Eudes-Deslongchamps, Reference Eudes-Deslongchamps1849 by original designation.
?Leptomaria sp.

Figure 5 Cretaceous Gastropoda recovered from Batavia Knoll. (1, 2) ?Leptomaria sp., WAM 15.102: (1) apical view; (2) adapertural view. (3–9) Planolateralus acanthanodus n. sp.: (3–6) adult specimen, WAM 15.143: (3) apical view; (4) apertural view; (5) adapertural view; (6) abapical view. (7–9) Oblique view of juvenile specimen, WAM 15.177: (7) showing basal ornamentation, umbilicus, and aperture details; (8, 9) detail of protoconch. (10) Turritella sp., apertural view, WAM 15.154. (11–16) Igonoia levimargarita n. sp. (11, 12) WAM 15.114: (11) apical view; (12) apertural view. (13) Adapertural view, WAM 15.132. (14) Abapical view, WAM 15.144. (15, 16) Detail of protoconch, WAM 15.132. (17) Cirsocerithium sp., apertural view, WAM.147. (18, 19) Procerithium arenacollicola n. sp., WAM 15.136: (18) apertural view; (19) adapertural view. All specimens are casts and coated with ammonium chloride. (1–6, 11–14, 18, 19) Scale bar=1 cm; (8, 9, 15, 16) scale bars=1 mm; (7, 10, 17) scale bar=5 mm.
Description
Last whorl of average size for the family, 40.7 mm in diameter. Last whorl is subcircular, and the large central void (umbilicus) is suggestive of a strongly phaneromphalous shell when complete. Whorl profile is slightly convex, with rounded periphery. No ornamentation is discernible, apart from possible weak orthocline growth lines.
Materials
Single fragmentary last whorl mold.
Remarks
This specimen is provisionally assigned to Leptomaria due to its convex, rounded whorl margins. Other pleurotomariid genera usually exhibit greater angularity in their whorl margins. The lack of external sculpture, including the position and form of the selenizone, prohibits more specific identification. Given the fragility of the shells of this family, it is reasonable to assume that the gastropod shell broke apart, perhaps during the mass-flow event that created the Batavia Knoll sandstone, leaving this whorl isolated and fractured.
According to a review of the genus by Monari and Gatto (Reference Monari and Gatto2013), Leptomaria ranged from the Aalenian (Middle Jurassic) to lower Cenomanian (Late Cretaceous) in Europe, from the Pliensbachian to Toarcian (Early Jurassic) in Argentina, and was found during the Callovian (Middle Jurassic) in southern India.
Superfamily Eucycloidea Koken, Reference Koken1896
Family Calliotropidae Hickman and McLean, Reference Hickman and McLean1990
Genus Planolateralus Sohl, Reference Sohl1960
Type species
Calliomphalus argenteus Wade, Reference Wade1926 by original designation.
Planolateralus acanthanodus new species
Holotype
WAM 15.143, an external mold; Batavia Knoll, eastern Indian Ocean; late Albian.
Diagnosis
Large Planolateralus, with deep, channeled sutures; whorls ornamented by up to six sharply nodulate spiral ribs, the fifth of which (from the apical suture) is dominant.
Occurrence
Batavia Knoll, eastern Indian Ocean; Early Cretaceous (late Albian).
Description
Trochiform shell, large size for genus (reconstructed height ~17 mm), of at least three teleoconch whorls, sides straight to slightly convex. Protoconch not preserved. Early whorls slightly convex, becoming slightly uni- to biangular on appearance of spiral ornament (two riblets) on second preserved whorl. Narrowly phaneromphalous. Spiral angle of 54º. Sutures deeply channeled, angled at 12º. Teleoconch sculpture consists of strong spiral nodulate ribs, three per whorl in early whorls (central rib strongest), increasing to six per whorl by penultimate whorl. Interstices subequal in width to spiral ribs, devoid of spiral ornament. On later whorls, two spiral ribs (third and fifth from apical suture) maintain greater strength than others until last whorl when third rib weakens to be of equal strength with others; fifth rib remains dominant. Ribs obliquely crossed by strong prosocline growth lines. Last whorl develops an abapical weak nodulate keel, demarcating the slightly convex base. Base ornamented by at least nine equidistant spiral cords on adult shell; juvenile shell possesses three beaded spiral cords on base, crossed by numerous fine axial threads (growth lines). Umbilicus narrow, with crenulated margin, interior of umbilicus ornamented with evenly spaced axial riblets. Aperture rounded to subquadrate, inner lip smooth, columella slightly twisted.
Etymology
From Latin acanthus (spine) and nodus (knot/node), referring to the shell’s sharp nodulate ornamentation.
Materials
Single entire mold and fragment of adult shell, six molds of juvenile shells.
Remarks
Of congeneric taxa, Planolateralus acanthanodus is probably most similar to C. argenteus Wade, Reference Wade1926 (p. 179, pl. 55, figs. 4–7, 11), particularly the subspecies C. (P.) argenteus spinosum Sohl, Reference Sohl1960 (p. 56, pl. 5, figs. 19, 23–25), from the Ripley Formation (Maastrichtian) of Tennessee. Planolateralus argenteus spinosum bears a well-developed, stronger, nodulate spiral rib near to the abapical suture on its later whorls, consistent with P. acanthanodus. The Batavia Knoll species, however, has deeper interstices between the spiral cords and displays sharper nodes than either Wade’s or Sohl’s figured specimens.
It is probable that the reduced sharpness of the spiral nodes in the adult shell herein, and the corded yet apparently unnoded base, are the result of cumulative abrasion over the life of the snail. It is also possible that these features are due to taphonomic factors.
Superfamily Trochoidea Rafinesque, Reference Rafinesque1815
Family Margaritidae Thiele, Reference Thiele1924
Genus Igonoia Squires, Reference Squires2011
Types species
Igonoia onoensis Squires, Reference Squires2011 by original designation.
Igonoia levimargarita new species
Holotype
WAM 15.114, an external mold; Batavia Knoll, eastern Indian Ocean; late Albian.
Diagnosis
Large Igonoia, bearing up to seven fine beaded ribs on apical surface (subsuture) of whorls; whorl sides smooth or displaying subtle prosocline growth lines.
Occurrence
Batavia Knoll, eastern Indian Ocean; Early Cretaceous (late Albian).
Description
Shell large for genus at an average of ~14 mm high, turbiniform, and phaneromphalous. Protoconch conical with one to two convex whorls. Five to six convex whorls in teleoconch. Spire 45% to 50% of total shell height. Pleural angle 80º to 81º and sutural angle 9º to 10º. Sutures impressed, slightly canaliculate. Protoconch and early teleoconch whorls devoid of sculpture; ornamentation begins about fourth whorl with two subsutural spiral ribs of equal strength. The number of subsutural ribs increases with growth, seven present on last whorl. Spiral ribs weaken anteriorly and become rapidly indistinct, leaving whorl sides almost smooth apart from weak prosocline growth lines. Intersection of growth lines and ribs results in beaded texture. Ornament on base consists of ~20 closely spaced spiral ribs, becoming broader and beaded toward umbilicus, resulting in cancellate texture. Umbilicus wide and deep, ornamented with faint reticulated texture. Rim of umbilicus angular and demarcated by moderately strong crenulate spiral rib. Aperture subcircular with thin outer lip.
Etymology
From Latin levis (fine/delicate) and margarita (bead/pearl), referring to the subtle, weakly developed beaded ornamentation on the teleoconch whorls.
Materials
Three external molds.
Remarks
The species described here is most similar to the late Albian species Igonoia kieli Squires, Reference Squires2011, of which it is undoubtedly phylogenetically allied. The specimens recorded by Squires (Reference Squires2011) in his description of that species are generally smaller than the ones here—no more than 9 mm in height and slightly wider than high. Specimens from Batavia Knoll are marginally taller than wide and reach a reconstructed height of almost 18 mm, though average approximately 14 mm. The ornamentation of the Batavia Knoll specimens is subtler than that of the specimens recorded by Squires (Reference Squires2011) and consists of a greater number of spiral ribs. These specimens also do not exhibit the concave ramp of I. kieli. The pleural angle of these specimens is also sharper (81º compared to I. kieli’s 87º). The differences here, as well as the paleogeographic distance between recorded species in northwestern North America and the eastern Indian Ocean, warrant the erection of a new species within Igonoia for the Batavia Knoll specimens.
Subclass Caenogastropoda Cox, Reference Cox1960
Superfamily Cerithioidea Fleming, Reference Fleming1822
Family Procerithidae Cossmann, Reference Cossmann1906
Subfamily Procerithiinae Cossmann, Reference Cossmann1906
Genus Procerithium Cossmann, Reference Cossmann1902
Type species
Procerithium quinquegranosum Cossmann, Reference Cossmann1902 by original designation.
Procerithium arenacollicola new species
Holotype
WAM 15.136, cast and external mold (with spire broken and steinkern preserved); Batavia Knoll, eastern Indian Ocean; late Albian.
Diagnosis
Average- to moderately large–sized procerithiid ornamented with two smooth, pointed, nodulate spiral ribs, located centrally and abapically on the whorl, asymmetrically aligned, with fine spiral striae between.
Occurrence
Batavia Knoll, eastern Indian Ocean; Early Cretaceous (late Albian).
Description
High-spired turriform shell composed of at least seven slightly convex whorls with a spiral angle of 18º to 20º. Average sized for genus (reconstructed height ~65 mm). Suture weakly impressed and canaliculate, almost flush, with angle of 9º to 10º. Ornamentation of two nodulate spiral ribs; one central and one abapical near the suture, with fine secondary interstitial spiral striations (approximately four per millimeter) between. Tubercles pointed and devoid of sculpture. Nodules on central rib markedly stronger than those lower on whorl. Tubercles asymmetrically aligned. Base of last whorl exhibits additional, slightly tuberculated rib, forming a weak keel. Aperture not well preserved, though appears subcircular with slight angular margins. Anterior canal missing. Apex broken in all specimens, inhibiting protoconch description.
Etymology
From Latin arena (sand), collis (hill), and incola (resident), referring to both its sandy-substrate life environment and preservation in sandstone on Batavia Knoll.
Materials
Four external molds, two almost complete and two fragmentary.
Remarks
The generic assignment of this species is difficult, as no specimen recovered preserves the protoconch. In terms of gross morphology, it is closest to either Procerithium Cossmann, Reference Cossmann1906 or Cryptaulax Tate, Reference Tate1869, two genera whose similarity is so great that it has been suggested they were based on the same species (Bandel, Reference Bandel2006). Ferrari (Reference Ferrari2012) argues, however, that certain distinctions can be made between the genera. In the absence of a protoconch, we rely on the diagnostic patterns of teleoconch ornamentation, as described by Ferrari (Reference Ferrari2012), to place this species within Procerithium according to the following criteria: the shell is nonturriculate in outline; axial ornamentation is not well developed on the teleoconch and disappears in mature whorls; and the ornamentation of mature whorls is spiral, noded, and includes distinct secondary spiral striae.
This species appears similar to the rare Cerithium limbatum described by Stoliczka (Reference Stoliczka1868, p. 194, pl. 15, figs. 13, 14; non Deshayes, Reference Deshayes1864) from the Ariyalur Group (Santonian to Maastrichtian) of southern India. Cerithium limbatum possesses two nodulate ribs of even strength, unlike the differing strength of ribs on this specimen. It also exhibits stronger axial ornamentation and a slightly undulating suture. Considering Stoliczka’s (Reference Stoliczka1868) specimens were recovered from Upper Cretaceous strata, it is possible the species described here is ancestral to C. limbatum. The ornamentation of this species is similar to ?Metacerithium sp. of Kiel and Bandel (Reference Kiel and Bandel2004, p. 118, fig. 7C) from the Cenomanian of Germany, which may be conspecific with C. limbatum from India and thus may indicate that this species belongs in Campanilidae Douvillé, 1904.
Subfamily Paracerithiinae Cossmann, Reference Cossmann1906
Genus Cirsocerithium Cossmann, Reference Cossmann1906
Type species
Cerithium subspinosum Deshayes in Leymerie, Reference Leymerie1842 by original designation.
Cirsocerithium sp.
Figure 5.17
Description
Slightly small for genus (height 7.8 mm), high-spired shell composed of three biconcave, uniangular whorls. Spiral angle 44º. Sutures impressed. Ornamentation of strong, evenly spaced prosocline axial ribs and a prominent single spiral carina. Sharp nodules at intersection of axial and spiral sculpture. Secondary spiral ribs present midway between central carina and sutures, marginally stronger on adaxial half of whorl, and abutting sutures. Last whorl possesses strong peribasal keel. Base strongly concave and adorned with sigmoidal axial lirae, connecting to axial ribs on teleoconch, and fine spiral striations. Aperture obliquely ovate, descending into a short, straight anterior canal.
Materials
Single external mold.
Remarks
The Batavia Knoll specimen differs from other Cirsocerithium by the strength of its peribasal keel (usually far more moderate) and concavity of its base (normally ranging from weakly convex to concave). It also possesses fewer teleoconch whorls (three, compared to the typical five or more) than other species in the genus. Its ornament is similar to the type species, C. subspinosum, though it has far fewer secondary spiral cords and the nodules on the whorls are much sharper than seen in C. subspinosum. Regarding the basal characteristics, C. collignoni Kiel, Reference Kiel2006 (p. 459, fig. 3.11–3.14) is nearest in form to the species described here, possessing a relatively sharp keep and concave base. The ornamentation of C. collignoni, however, differs too significantly for it to be considered conspecific.
Superfamily Turritelloidea Lovén, Reference Lovén1847
Family Turritellidae Lovén, Reference Lovén1847
Genus Turritella Lamarck, Reference Lamarck1799
Description
Small, high–spired turriform shell composed of at least three orthogonal whorls with a spiral angle of ~12º. Suture flush to slightly impressed. Ornamentation of four evenly spaced spiral ribs; abapical cord, abutting suture, is unadorned, all others weakly nodulate; second from abapical suture is marginally stronger than other nodulate ribs (all of which are stronger than anteriormost rib); fine secondary unnoded spiral striations in interspaces. Tubercles asymmetrically aligned. Apex and later whorls unknown.
Materials
Single fragmentary mold.
Remarks
Only three minute volutions of a single individual of this species have been recovered from Batavia Knoll. Several mid-Cretaceous Turritella species bear nodulate ornamentation, so without a more complete specimen or better preservation, it is difficult to assign the species here to any one previously documented with any conviction. It is probably most similar to T. infralineata Gabb, Reference Gabb1864, from the late early Albian of northern California. The Batavia Knoll specimen exhibits weakly noded spiral ribs with relatively wide interspaces containing unadorned riblets, features seen in T. infralineata. The spire angle of the specimen here (12º) is similar to that of T. infralineata (11º). It differs in the strength of ribs across the whorl, however, particularly those abutting the sutures. According to Squires and Saul (Reference Squires and Saul2006), the posteriormost rib is of equal strength to the others in T. infralineata, whereas in the specimen here it is somewhat reduced.
Order Littorinimorpha Golikov and Starobogatov, Reference Golikov and Starobogatov1975
Superfamily Stromboidea Rafinesque, Reference Rafinesque1815
Family Aporrhaidae Gray, Reference Gray1850
Genus Anchura Conrad, Reference Conrad1860
Type species
Anchura abrupta Conrad, Reference Conrad1860 by monotypy.
Anchura pelsaerti new species

Figure 6 Cretaceous Gastropoda, Scaphopoda, Cephalopoda, Ostracoda, Annelida, and Echinoidea recovered from Batavia Knoll: (1–4) Anchura pelsaerti n. sp., WAM 15.113. (1) apertural view; (2) apical view; (3) adapertural view; (4) lateral view. (5–7) Drepanocheilus bataviensis n. sp.: (5) adapertural view, WAM 15.119; (6) apertural view, WAM 15.112; (7) apertural view of a somewhat abraded specimen, showing full habit of wing, WAM 15.145. (8, 9) ?Pseudomalaxis sp., WAM 15.121: (8) apical view; (9) adapertural view. (10, 11) ?Architectonica sp. whorl fragment, WAM 15.122, showing (10) umbilical and (11) external ornamentation. (12) Avellana sp. cf. A. subincrassata d’Orbigny, Reference d’Orbigny1850, apertural view, WAM 15.181. (13) Dentalium sp., WAM 15.134. (14) Echinoid? fragment, WAM 15.117. (15–17) Planohamites sp. (15, 16) WAM 15.149: (15) dorsal view; (16) transverse view. (17) Impression of lateral surface, WAM 15.182. (18) Parsimonia ootatoorensis (Stoliczka, Reference Stoliczka1873), from surface of the boulder; partial dissolution of calcareous wall reveals growth lines as sharp ridges, WAM 15.101. (19, 20) Bhimaites sp. cf. B. stoliczkai Kossmat, Reference Kossmat1898, dorsal views, WAM 15.142. (21, 22) Trachyleberididae gen. indet. sp. indet., WAM 15.163: (21) left carapace; (22) ventral view. (23, 24) ?Spinoleberis sp., WAM 15.161: (23) left carapace; (24) ventral view. (1–16, 19, 20) Specimens are casts; (1–20) specimens coated with ammonium chloride. (1–20) Scale bar=1 cm; (21–24) Scale bars=200 µm.
Holotype
WAM 15.113, cast and external mold; Batavia Knoll, eastern Indian Ocean; late Albian.
Diagnosis
Small Anchura possessing unique adapertural digitation on rear of wing; teleoconch ornamented with both spiral and radial elements, the latter diminishing abapically, and the former characterized by a strong subcentral carina between weaker cords.
Occurrence
Batavia Knoll, eastern Indian Ocean; Early Cretaceous (late Albian).
Description
Strongly alate aporrhaid, small for genus (maximum height 32 mm). Spiral angle 35º. Sutures impressed. Protoconch unknown. Inflation moderate and relatively uniform from early teleoconch whorls to last whorl. Growth lines prosocline and opisthocyrt below whorl midpoint, and opisthocline and opisthocyrt above. Last whorl strongly biangulate, bearing strong subcentral smooth carina and equally strong peribasal keel above onset of strong basal constriction. Ornament of last whorl consists of three secondary spiral cords, two above and one below the subcentral carination; below peribasal keel, whorl is ornamented with ten subequally spaced spiral threads. Moderately high spire of six convex whorls, occupying approximately 45% of total height. First visible whorl smooth, later spire whorls bear reticulate ornament. Transverse element consists of subequidistant ribs, arising on second visible whorl, decreasing in strength anteriorly, becoming obsolete on penultimate whorl. Spiral element primarily consists of a strong subcentral cord and slightly weaker cord midway between subcentral cord and abapical suture. Secondary spiral ornament of three subequally spaced weak cords posterior to subcentral carination, and a single cord adjacent to abapical suture. Smooth between cords, no interstitial threads. Aperture elongate and teardrop shaped, rounded posteriorly and drawn out into a long, narrow anterior canal, which follows left-curving rostrum. Inner lip with moderately thick callus pad, slightly raised from last whorl at apical limit, forming thin ridge to posterior of aperture. Outer lip slightly callused, expanding to a strongly adaxially developed wing. Wing does not encroach on penultimate whorl and extends orthogonally into an elongate spur reinforced by the spiral carination of the body, with a corresponding incised groove on the interior surface. Shaft divides into two thin spurs approximately 5 mm along length (in type), with posterior spur curving apically. Both spurs are broken, so extent and habit unknown. Minor rounded digitation occurs on the outer lip, approximately halfway between spur and rostrum, projecting anteriorly. Adapertural surface of wing possesses additional short (approximately 2 mm in length on type) blunt projection, formed at the junction of the peribasal keel of the body and ridges running from the rostrum and along base of shaft. Abapical and adaxial of this digitation, between the basal spur ridge and rostral ridge, the wing is devoid of ornamentation.
Etymology
For Francisco Pelsaert (d. 1630), commander of the Dutch East India Company trading ship Batavia, for which the knoll is named.
Materials
Single external mold, designated holotype.
Remarks
A single specimen was recovered in good preservation state. Ornamentation of this species is not perfectly comparable to any currently described species of Anchura, and the third, adaperturally projecting digitation is not previously recorded for the genus. Among smaller Anchura species, it is probably closest in morphology to the early Campanian A. halberdopsis Elder and Saul, Reference Elder and Saul1996 (p. 384, fig. 3.1–3.4), from which it differs by the spiral, rather than nodulate, sculpture on the body whorl, the penultimate whorl-encroaching wing, and the digitation pattern on the wing.
Genus Drepanocheilus Meek, Reference Meek1864
Type species
Rostellaria americana Evans and Shumard, Reference Evans and Shumard1857 (=Drepanocheilus evansi Cossmann, Reference Cossmann1904) by original designation.
Drepanocheilus bataviensis new species
Holotype
WAM 15.112, cast and external mold; Batavia Knoll, eastern Indian Ocean; late Albian.
Diagnosis
Small Drepanocheilus possessing fine spiral sculpture and devoid of axial ornamentation.
Occurrence
Batavia Knoll, eastern Indian Ocean; Early Cretaceous (late Albian).
Description
Small for genus (height of reconstructed shell maximum 25 mm), shell moderately thick, strongly alate. Spiral angle 35º to 38º, sutural angle 9º. Sutures impressed. Protoconch unknown. Teleoconch inflation moderate and uniform to last whorl. Growth lines shallowly opisthocline. Last whorl strongly uniangulate, bearing strong subcentral non-tubercle–bearing carina, and poorly developed peribasal keel just below carina and above onset of strong basal constriction. Last whorl ornamented with at least 10 spiral cords, decreasing in strength away from carina. Spire relatively high, occupying approximately 45% of total height and consisting of six strongly convex whorls. Spire whorls ornamented with six subequally spaced spiral cords that increase in strength away from protoconch. Spiral cords weakly developed above midpoint of each whorl. Smooth between cords, with no interstitial threads. No axial sculpture. Ramp angular on early whorls, becoming convex on later whorls. Aperture elongate, sublenticular to subovate with narrow notch, extending into a short, straight anterior canal. Inner lip with thick callus pad, extending from base of siphonal canal to antepenultimate whorl. Outer lip moderately callused, expanding to a strongly adaxially developed wing. Wing encroaches on lower part of penultimate whorl and extends into an elongate, weakly curved, tapering spike reinforced by the spiral carination of the body, with a corresponding incised groove on the interior surface. Small lobe on anterior margin of wing. Length of spike subequal to height of spire.
Etymology
For its occurrence on Batavia Knoll.
Materials
Three fragmentary molds (WAM 15.124, 15.129, and 15.158).
Remarks
This specimen was assigned to Drepanocheilus due to the form of the anterior canal and outer lip (Sohl, Reference Sohl1960; Kollmann, Reference Kollmann2009). The lack of transverse sculpture in this species is anomalous among Drepanocheilus, a genus usually characterized by strong axial ornamentation. This is the only currently documented species of Drepanocheilus to exhibit spiral threads to the complete exclusion of axial sculpture. This lack of axials is consistent over all specimens recovered, so is unlikely to be the result of abrasion on the shell removing such sculpture. On comparison with the type species for this genus, D. evansi, apart from the aforementioned lack of axial sculpture, this species possesses a thicker callus pad on the inner lip, as well as a more pronounced lobe on the anterior margin of the wing. This species is most similar to D. herberti Kiel and Bandel, Reference Kiel and Bandel2002 from the Santonian of South Africa, though that species is smaller (~11 mm compared with ~23 mm height), possesses more angular late teleoconch whorls, and maintains some axial sculpture through its entire height.
Subclass Heterobranchia Burmeister, Reference Burmeister1837
Superfamily Acteonoidea d’Orbigny, Reference d’Orbigny1843
Family Ringiculidae Fischer, Reference Fischer1883
Genus Avellana d’Orbigny, Reference d’Orbigny1842
Type species
Avellana avellana Brongniart, 1822 in Cuvier and Brongniart, Reference Cuvier and Brongniart1822 by tautonomy.
Avellana sp. cf. A. subincrassata d’Orbigny, Reference d’Orbigny1850
1842 cf. Avellana incrassata d’Orbigny, p. 133, pl. 168, figs. 13–16.
1850 cf. Avellana subincrassata d’Orbigny, p. 128.
2015 cf. Avellana subincrassata; Reference Ayoub Hannaa, Radulović, Radulović and FürsichAyoub Hannaa et al., p. 56, figs. 14G–14J (further synonymy therein).
Occurrence
Europe; Early Cretaceous (Albian) to Late Cretaceous (Turonian).
Description
Shell globular, subovate, average size for genus (height 21.8 mm; width 16.1 mm). Low-spired teleoconch of one or two subtrapezoid, slightly convex whorls; spire somewhat gradate, pleural angle 76º. Inflation moderate in spire, then rapid between penultimate and final whorl. Protoconch not preserved. Sutures moderately impressed, slightly canaliculate. Ornamentation of evenly spaced spiral ribs (at least 31 on body whorl) crossed by thin evenly spaced orthocline axial lirae (four to five per millimeter), resulting in fine clathrose texture; spiral cords three to four times thicker than axials. Aperture holostomatous, teardrop shaped, well rounded anteriorly; labrum strongly thickened; callus projecting approximately halfway up visible surface of penultimate whorl; inner side smooth.
Materials
One mold, with steinkern.
Remarks
The poor preservation of apertural characteristics severely inhibits the accurate identification of this species, as the columella and inner labral features are key in distinguishing between Cretaceous ringiculids (see Squires and Saul, Reference Squires and Saul2001; Stilwell and Henderson, Reference Stilwell and Henderson2002 for brief discussions on the generic significance of these characteristics). On the Batavia Knoll specimen, the columellar folding is not preserved or not observable, nor is it apparent whether the inner surface of the thickened outer lip bears any dentition. The presence and position of an apertural notch is also unknown. Despite these, the specimen conforms well to Avellana subincrassata in its size, dimensions, and ornamentation.
Superfamily Architectonicoidea Gray, Reference Gray1850
Family Architectonicidae Gray, Reference Gray1850
?Architectonicidae gen. indet. sp. indet.
Description
Of average to slightly small size for family (12 mm basal diameter). Markedly phaneromphalus; umbilicus is approximately one-third the diameter of the whorl. Whorl profile is trapezoidal, with rounded basal periphery. Apical surface of single preserved whorl maintains a smooth concentric depression, indicating position of preceding whorl and impressed sutures. Aperture absent. Whorl ornamentation consists of thin, evenly spaced, spiral and axial striations, resulting in a weak reticulate texture. Base ornamented with predominantly axial striations, forming crenulations on meeting the angular umbilical margin. Umbilicus displays same reticulate texture as exterior, though spiral elements are marginally stronger.
Materials
Single last whorl fragmentary mold.
Remarks
This specimen is provisionally assigned to Architectonicidae due to its compressed and highly umbilicate form with a trapezoid whorl profile. It possesses ornamentation similar to that of ‘Solarium’ vylapaudiense Stoliczka, Reference Stoliczka1868 (p. 257, pl. 20, figs. 5, 6) from the Sillakkudi Formation of southern India (Santonian to Campanian), though S. vylapaudiense possesses a strong carination on its body whorls. Indeed, most compressed architectonicid genera exhibit a moderately sharp to strong peribasal keel, which is not seen in the Batavia Knoll specimen. This may indicate that it should instead be considered a member of the Solariellidae (Trochoidea), which also embraces low-spired, compressed forms. The whorl profiles of solariellids, however, are generally well rounded and evenly convex, as opposed to the angularity displayed by this species.
Genus Pseudomalaxis Fischer, Reference Fischer1885
Description
Shell large for genus, discoidal, broadly umbilicate (umbilicus ~50% of diameter). Spire flat, suture slightly impressed. Whorl margins subquadrate. Coarse sculpture of spiral noded ribs, six per whorl. Rib proximal to outside whorl limit is strongest, giving margin a crenulated appearance. Inside this rib is a weak rib succeeded by a stronger rib, followed by three weaker ribs of equal strength to the second, all of which are noded due to the intersection of axial striae. Lateral whorl surfaces are similarly ornamented, with at least seven spiral beaded ribs of equal strength.
Materials
Single fragmentary mold, with some original shell preserved.
Remarks
The subquadrate whorl margins of this specimen are characteristic of Pseudomalaxis and its close relatives, though they are usually on the order of a few millimeters in diameter, making this quite a large specimen for the group. The provisional assignment here is due to the poor preservation of the specimen, obscuring much detail, including the protoconch. In addition, this genus is so far unknown from Lower Cretaceous rocks. Pseudomalaxis is a predominantly Quaternary genus, with Bieler and Petit (Reference Bieler and Petit2005) suggesting it definitively only ranges down to the Pliocene. In their catalog of Architectonicidae genera, however, they maintain Cretaceous species as valid members of the genus. The earliest of these, Pseudomalaxis pateriformis Stephenson, Reference Stephenson1955, is documented from Campanian strata of the southeastern United States (Dockery, Reference Dockery1993). Cladistic studies conducted by Bieler (Reference Bieler1988) indicate the Pseudomalaxis/Spirolaxis Monterosato, Reference Monterosato1913 group within the architectonicids diverged from the rest of the family in the Cretaceous, before the divergence of both Pseudotorinia Sacco, Reference Sacco1892, and Heliacus d’Orbigny, Reference d’Orbigny1842/Solatisonax Iredale, Reference Iredale1931 clades. Heliacus is well documented from Campanian and younger rocks (e.g., Dockery, Reference Dockery1993), so it stands to reason that Pseudomalaxis, which diverged first, is found in older strata than Heliacus.
The sculpture of P. pateriformis is much finer than that of the Batavia Knoll specimen, with spiral lirae across the whorls (Dockery, Reference Dockery1993, pl. 34, figs. 6–11), rather than the beaded sculpture of the species described herein. Of interest, P. pateriformis is also a larger-than-average member of the genus (holotype 13 mm in diameter; Stephenson, Reference Stephenson1955), perhaps suggesting a pattern of size reduction within the genus over time.
Class Scaphopoda Bronn, Reference Bronn1862
Order Dentaliida da Costa, Reference da Costa1778
Family Dentaliidae Gray, Reference Gray1847
Genus Dentalium Linnaeus, Reference Linnaeus1758
Type species
Dentalium elephantium Linnaeus, Reference Linnaeus1758 by subsequent designation (de Montfort, Reference de Montfort1810).
Dentalium sp.
Description
Shell weakly curved, tapering, of average to small size for family (reconstructed fragment length 36.7 mm). Aperture broken. Apex possibly exhibits two or three lateral notches. Circular in cross section at apex, becoming elliptical aperturally. No clear ornamentation visible on exterior, though possible longitudinal ribs and oblique striae present.
Materials
One fragment in two parts, mold and steinkern.
Remarks
Only a single dentaliid test of significant size was recovered, with a few possible impressions noted in the rock also. Poor preservation inhibited a more specific identification, and more certain description, of this specimen. Aperture of the specimen is missing, so full length is unknown. Overall, its size, degree of curvature, and longitudinal ornamentation place it within Dentalium, some species of which do exhibit lateral notches at their apices (Lamprell and Healy, Reference Lamprell and Healy1998). The Southern Hemisphere Cretaceous scaphopod record is extremely poor, with only 19 species identified from Aptian to Maastrichtian strata, exhibiting a high level of endemicity throughout the period (Stilwell, Reference Stilwell1999; Stilwell and Henderson, Reference Stilwell and Henderson2002). Of these, only six are known from mid-Cretaceous (Aptian to Turonian) deposits, and merely two of those are ascribable to Dentalium. Dentalium sp. A and Dentalium sp. B of Stilwell (Reference Stilwell1999) are recorded from Aptian and Cenomanian rocks, respectively. Neither of these is conspecific with the Batavia Knoll species, the primary difference being the ovate nature of the species here toward its aperture. Given the highly endemic character of Cretaceous Scaphopoda, and the significant paleodistances between known coeval species, it may be necessary to erect a new species to accommodate the Batavia Knoll specimen as new specimens come to light.
Class Cephalopoda Cuvier, Reference Cuvier1797
Subclass Ammonoidea von Zittel, Reference von Zittel1884
Order Ammonitida Hyatt, Reference Hyatt1889
Suborder Ammonitina Hyatt, Reference Hyatt1889
Superfamily Desmoceratoidea von Zittel, Reference von Zittel1895
Family Desmoceratidae von Zittel, Reference von Zittel1895
Subfamily Puzosiinae Spath, Reference Spath1922
Genus Bhimaites Matsumoto, Reference Matsumoto1954
Type species
Ammonites bhima Stoliczka, 18Reference Stoliczka65 by original designation.
Bhimaites sp. cf. B. stoliczkai Kossmat, Reference Kossmat1898
1865 cf. Ammonites beudanti Reference StoliczkaStoliczka, p. 142, pl. 71, figs. 1–4, pl. 72, figs. 1, 2.
1898 cf. Puzosia stoliczkai Kossmat, p. 119, pl. 24, fig. 6.
2014 cf. Puzosia (Bhimaites) stoliczkai; Kennedy and Klinger, p. 10, fig. 11a–c (full synonymy therein).
Occurrence
Spain, France, Persia, northern South America, southern Africa, Madagascar, southern India; Early Cretaceous (late Albian) to Late Cretaceous (early Cenomanian).
Description
Small for genus (approximately 30 mm in whorl diameter), subinvolute and laterally compressed with rounded venter and slightly concave flanks. Surface ornamentation of poorly developed growth lines and relatively widely spaced ribs. Suture trace is complexly ammonitic and has a dendritic profile.
Materials
Single fragmentary mold and several steinkern (chamber) fragments.
Remarks
Due to the incomplete nature of the material and suboptimal preservation of sutural contacts, doubt is cast onto the identification of this specimen. It does, however, conform well to Bhimaites stoliczkai in its dimensions, degree of compression, and minimal external ornamentation. It represents a rather small individual, as the species generally reaches an excess of 70 mm in diameter, though specimens of this size have been documented (e.g., Collignon, Reference Collignon1961).
Suborder Ancycloceratina Wiedmann, Reference Wiedmann1966
Superfamily Turrilitoidea Gill, Reference Gill1871
Family Hamitidae Gill, Reference Gill1871
Genus Planohamites Monks, Reference Monks2002
Type species
Hamites compressus Sowerby, Reference Sowerby1814 by original designation.
Planohamites sp.
Description
Average size for genus. Shaft straight to slightly bent, with compressed, ovate cross section (whorl breadth:whorl height=0.75), possibly tapering to a slight keel along venter. Ornamentation of numerous fine concentric ribs, approximately one per millimeter (eight ribs in 10 mm), with interstices slightly wider than the ribs. Suture ammonitic with subquadrate profile.
Materials
Single mold with steinkern, with possible fragments (whether of the same or separate individuals is unknown), two impressions of the lateral surface of the shaft.
Remarks
The compressed transverse whorl section and closely spaced ribs place this species within Planohamites Monks, Reference Monks2002, though the poor preservation and fragmentary nature of specimen(s) inhibit more specific identification. The sutural trace is inconclusive as only a general pattern can be discerned and it could be applied to several genera in a number of families. The ribs of this specimen are finer and subtler than is typical for the genus, and the apparent weak keel is also an anomalous feature.
Phylum Annelida Lamarck, Reference Lamarck1809
Class Polychaeta Grube, Reference Grube1850
Subclass Canalipalpata Rouse and Fauchald, Reference Rouse and Fauchald1997
Order Sabellida Fauchald, Reference Fauchald1977
Family Serpulidae Rafinesque, Reference Rafinesque1815
Genus Parsimonia Regenhardt, Reference Regenhardt1961
Type species
Parsimonia parsimonia Regenhardt, Reference Regenhardt1961 by original designation.
Parsimonia ootatoorensis (Stoliczka, Reference Stoliczka1873)
1873 Serpula ootatoorensis Stoliczka, p. 64, pl. 12, figs. 9, 10.
non1875 Serpula ootatoorensis, Reference von Salvini-PlawenGeinitz, p. 283, pl. 63, figs. 4, 5.
1959 Serpula ootatoorensis; Reference Besairie and CollignonBesairie and Collignon, p. 199.
1973 Parsimonia ootatoorensis (Stoliczka); Reference Chiplonkar and TapaswiChiplonkar and Tapaswi, p. 121, pl. 8, fig. 8.
Holotype
GSI 01840 (Stoliczka, Reference Stoliczka1873, pl. 12, fig. 9); Uttatur Group, southern India; Albian to Cenomanian.
Occurrence
Southern India, Madagascar; late Albian to Cenomanian.
Description
Straight to submeanderiform tube, moderate to slightly small for species (average diameter approximately 5 mm). Subcircular to subovate in cross section with subcircular, unadorned lumen. Tube wall ranging from thin to comparatively thick. Exterior surface ornamented by regular, concentric growth lines, but otherwise smooth.
Materials
Numerous tubes throughout the rock, one slightly dissolved specimen from surface of boulder.
Remarks
Parsimonia ootatoorensis is known from numerous specimens within the Batavia Knoll sandstone; however, the majority of these remain within the rock and have not been extracted. They primarily appear as single, isolated tubes (found in cross section during dissection of the boulder) though one cluster of 13 tubes was discovered. Due to their entombment within the rock, their general tube-building habits are unknown, as are their relationships with the substrate. Two instances of colonization of this serpulid on molluscan shells within the Batavia Knoll sandstone are documented; one on the external surface of the ?Pseudomalaxis sp. specimen, and several individuals were observed on the external and internal surfaces of a Procerithium arenacollicola n. sp. One tube was partially excavated from its block, which revealed an increase in diameter of that specimen from 4.4 mm to 6.3 mm over a length of approximately 40 mm.
The single occurrence of this species at the surface of the boulder (Fig. 6.18) preserves four tube sections, though whether they are attributable to single or multiple individuals is unknown. Partial dissolution of the internal layer of the calcareous tube here results in the growth lines being visible as a series of sharp concentric ridges on the interior surface.
Jäger (Reference Jäger2014) emended the identification from Geinitz (Reference Geinitz1875; and subsequent authors) of this species from upper Cenomanian rocks in Saxony, noting specimens there should correctly be ascribed to Sabella Linnaeus, Reference Linnaeus1767. This leaves P. ootatoorensis, with occurrences in Madagascar, southern India, and now Batavia Knoll, as being a purely Gondwanan annelid.
Phylum Arthropoda von Siebold, Reference von Siebold1848
Subphylum Crustacea Brünnich, Reference Brünnich1772
Class Ostracoda Latreille, Reference Latreille1802
Subclass Podocopa Sars, Reference Sars1866
Order Podocopida Müller, Reference Müller1894
Suborder Cytherocopina Baird, Reference Baird1850
Superfamily Cytheroidea Baird, Reference Baird1850
Family Trachyleberididae Sylvester-Bradley, Reference Sylvester-Bradley1948
Genus Spinoleberis Deroo, Reference Deroo1966
Type species
Cythere eximia Bosquet, Reference Bosquet1854 by original designation.
?Spinoleberis sp.
2009 ?Spinoleberis Babinot et al., p. 9, pl. 4, figs. 7, 8.
Description
Carapace average size for genus, at 0.75 mm long, subtrigonal in outline, anterior margin broadly rounded, thickened, and crenulated. Posterior margin narrower, convex, and crenulated. Dorsal margin straight, ventral margin slightly sinuous; margins subparallel, converging toward the posterior. Moderate inflation, having almost equal height to width of articulated carapace. Subcentral tubercle hemispherical and prominent on both valves. Left valve possesses a strong subcentral ridge with a tubercle. Right valve is devoid of this tubercle. Lateral surface appears smooth, excepting crenulations along anterior and posterior margins.
Materials
Single articulated shell and possible hinge fragment.
Remarks
This ostracod appears to be aligned with the Albian to Maastrichtian trachyleberidid genus, Spinoleberis Deroo, Reference Deroo1966, but Rehacythereis Gründel, Reference Gründel1973 or Cythereis Jones, Reference Jones1849 cannot be entirely discounted, although this specimen does not appear to possess the reticulate sculpture of the latter genera. This specimen is herein tentatively assigned to Spinoleberis due to its morphological affinity with specimens described by Babinot et al. (Reference Babinot, Colin and Randrianasolo2009) from mid to late Albian strata of Madagascar and provisionally assigned by them to the same genus. Due to its size, it is probable the specimen recovered from Batavia Knoll is male.
?Trachyleberididae gen. indet. sp. indet.
Description
Small carapace for family at just over 0.3 mm long, subtrigonal in lateral view, moderately inflated in dorsal view (subequal height and width). Anterior margin narrow and quadrate, posterior margin well rounded. Dorsal margin straight, ventral margin convex. Subcentral ridge connects to prominent orbicular tubercle on both valves. Second, slightly less prominent, tubercle sits ventral to orbicular tubercle on ventral margin. In ventral view, lateral margins angular and taper to a point. Surface is finely reticulated.
Materials
One articulated specimen.
Remarks
A single extracted ostracod, broadly similar in shape to ?Spinoleberis above, yet has a more convex ventral margin and appears to be reverse oriented, with the narrow end being anterior to the wider. Alternatively, the prominent tubercles could be located posteriorly, rather than anteriorly, as is typical. Either of these assertions calls into question the assignment of this specimen within the Trachyleberididae, which do not generally exhibit singular strong posterior tubercles or a narrow anterior margin.
Phylum Echinodermata Klein, Reference Klein1734
Class Echinoidea Leske, Reference Leske1778
Echinoidea ord. indet. fam. indet.
Description
Convex shell fragment ornamented predominantly by longitudinal striations. ‘Lower’ sector (approximately half the width of the fragment) slightly depressed from the ‘upper’ and striations thereon broadly sigmoidal, curving into the ‘point’ of the fragment. ‘Upper’ portion dominated by strong striations crosscutting longitudinal lines at approximately 50º from horizontal. Where these crosscutting striae meet the line of depression a slight crenulated ridge forms.
Materials
Isolated fragment, mold.
Remarks
The texture displayed by this shell fragment is not common among any molluscan class, resulting in its tentative identification as an echinoderm fragment. Echinoderm spines are evident in thin sections of the Batavia Knoll sandstone, indicating that this phylum did inhabit the area of deposition. The minute size of the echinoderm spines is indicative of irregular urchins, such as heart urchins and sand dollars. The angled texture of the ‘upper’ portion could be synonymous with ambulacral groove (petal) patterns. Heart urchins and sand dollars preferentially reside in sandy substrates (Chao, Reference Chao2000), which is consistent with the other fauna described herein from the Batavia Knoll sandstone.
Acknowledgments
We are indebted to the researchers from the University of Tasmania and University of Sydney (J. Whittaker, J. Halpin, S. Williams, et al.) who undertook the initial voyage to collect samples, especially to J. Whittaker for providing funding from her DECRA grant (DE140100376) to the authors for this research to be completed. Our thanks also go to S. Morton, of the Department of Physics, Monash University, for his phenomenal photography skills. For assistance and clarification of certain taxa, our gratitude goes to R. Squires (for Igonoia), E. Kupriyanova and A. Ippolitov (for the serpulid), P. Quilty (for Foraminifera), and C. Mays (for palynomorph grains). We also extend our thanks to editor M. Hautmann and reviewers S. Schneider and S. Kiel for their constructive feedback and improvement of this work.








