Introduction
The earliest credible clam shrimp eggs date back to Late Carboniferous times, and only three localities yielding fossil eggs have been recorded from the whole Paleozoic. One concerns the eggs of Estheria cebennensis from the lower Stephanian (Upper Carboniferous) sediments of Gagnières, France (Dechaseaux, Reference Dechaseaux1951, p. 127, fig. 1, pl. 1, figs. 1, 7–8; Feys, Reference Feys1985, pl. 2, fig. 5). The second description deals with eggs belonging to Montcestheria orri from the Late Carboniferous Montceau Lagerstätte, France (Vannier et al., Reference Vannier, Thiéry and Racheboeuf2003, p. 1014, pl. 4, figs. 8–13). The third record is from the Upper Oberhof Formation (early Permian) of Gotha, Germany, where eggs were found in Pseudestheria n. sp. A (Martens, Reference Martens1983, p. 62, pl. 3, fig. 4), Lioestheria pseudotenella (Martens, Reference Martens1983, p. 37, pl. 34, figs 1–4), and Limnolimnadia ilfeldensis (Martens, Reference Martens1983, p. 49, text-fig. 57; p. 105, pl. 46, figs 1–7). When it comes to the Mesozoic, there are more reliable records. Fossil eggs from four localities aged as Triassic has been documented: eggs of Estheria minuta from Voltz sandstones (Bill, Reference Bill1913, p. 326, fig. 2; Grauvogel, Reference Grauvogel1947); eggs of Estheria striata from northern France (Pruvost, Reference Pruvost1919); eggs of Palaeolimnadia alsatica (the same specimens as figured by Gall and Grauvogel [1966] but misidentified as Euestheria albertii) and eggs of Palaeolimnadia sp. by Gall (Reference Gall1971, p. 41, pl. 19, fig. 1); eggs of Palaeolimnadia sp from Kalkschieferzone of Lombardy, N. Italy (Tintori, Reference Tintori1990, p. 102, fig. 6). During the Jurassic, two localities yielding fossil eggs of clam shrimp have been reported: eggs of Euestheria luanpingensis from the Jiulongshan Formation of Zhouyingzi, Luanping County, Heibei Province (Zhang et al., Reference Zhang, Shen and Niu1987, p. 113, pl. 2, figs 1–4; Zhang et al. Reference Zhang, Shen and Niu1990, p.313, pl. 3, fig. 9, pl. 4, figs 1, 3–4, pl. 11, fig. 5), and from the Daohugou Fossil Beds of Daohugou, Ningcheng County, Inner Mongolia (Shen et al., Reference Shen, Chen and Huang2003, pl. 1, fig. 2; Shen and Huang, Reference Shen and Huang2008, p. 355, fig. 4A–G), in northeastern China. While in the Cretaceous, compared to the high diversity of clam shrimps, only one reliable record of fossil eggs has been reported: eggs of Palaeolynceus stschukini from the Lower Cretaceous of the East Transbaical, Russia (Trusova, Reference Trusova1974, pl. 1, fig. 2). Besides, additional fossil clam shrimp eggs are also briefly mentioned in some studies, but are not described in detail and poorly figured (e.g., Paleolynceus stchukini in Chernyshev [Reference Chernyshev1940]; Eosestheria middendorfii [Jones, Reference Jones1862]). Although frequently called eggs, these structures are actually dormant, encysted embryos (gastrula) (Dumont and Negrea, Reference Dumont and Negrea2002).
Martin (Reference Martin1989) pointed out that the morphology and size of extant clam shrimp eggs may be of systematic value, which has been verified by various studies (e.g. Belk, Reference Belk1989; Martin, Reference Martin1989; Mura, Reference Mura1991; Thiéry and Gasc, Reference Thiéry and Gasc1991; Rabet, Reference Rabet2010; Mura and Rossetti, Reference Mura and Rossetti2010; Zarattini et al., Reference Zarattini, Mura and Ketmaier2013; Bruner et al., Reference Bruner, Costantini and Mura2013). Shen and Huang (Reference Shen and Huang2008) discussed the morphological relationships of the Jurassic and extant clam shrimp eggs from China. Compared to various studies on the morphology of extant clam shrimp eggs, the shape and microstructures of the fossil eggs remain largely unknown.
Recently, numerous Eosestheria elliptica Chen, 1976 (in Zhang et al., 1976) specimens with three-dimensionally preserved eggs were collected from the Barremian to Aptian Yixian Formation, western Liaoning, China. These eggs display a spherical shape with smooth surface, part of the tertiary envelope and possibly the first embryonic cuticle, all features previously unknown or ambiguous. The material not only furthers our understanding of the biology of ancient clam shrimps, but also adds to our knowledge of the fossilization processes that are responsible for the exceptional preservation of the Jehol Biota.
Material and methods
Intact clam shrimp carapaces commonly occur in the Yixian Formation, and are by far the most dominant aquatic organism (e.g., Fürsich et al., Reference Fürsich, Sha, Jiang and Pan2007; Pan et al., Reference Pan, Sha, Fürsich, Wang, Zhang and Yao2012). In 2007, a high-resolution excavation was conducted at Erdaogou village, Beipiao County, western Liaoning (N41°31.942’, E120°47.747’). Abundant valves of the clam shrimp E. elliptica with eggs have been collected from layer S1 of the Erdaogou Section (for section details see Pan et al., Reference Pan, Sha, Fürsich, Wang, Zhang and Yao2012). In the collection, approximately 20% of the E. elliptica specimens contain eggs. These eggs are usually dispersed within an area of about 5×3 mm located in the middle of the valves, occasionally slightly closer to the ventral margin (Fig. 1.1, 1.2). The three-dimensional eggs are embedded in sediment between the lower valve and fragments of the upper valve (Fig. 1.3, 1.4).

Figure 1 Eosestheria elliptica Chen, 1976 and their eggs from the Early Cretaceous Yixian Formation, Erdaogou, Beipiao County, western Liaoning. (1, 2) Articulated valves with eggs at the middle part of the valves, NIGP 161062–161063; (3, 4) the SEM images of the eggs embedded within the sediments, NIGP 161064.
The hand specimens of E. elliptica collected in the field were first studied under the optical microscope, and one specimen with several eggs still in place was coated with gold and analyzed with the scanning electron microscope (SEM). To obtain a clearer image of the three-dimensional features, individual eggs were removed from the rock and cleaned. First, the surrounding sediment was moistened with water before a fine needle was used to loosen the adjacent matrix. Then the eggs, still with some sediment adhering to them, were transferred to a tray with the help of a fine brush and a drop of water, to which the eggs stuck. In a second step, the surface of the individual eggs was cleaned by treating them with diluted H2O2 (2.5%–3.5%) for approximately 2–3 hours in the tray. Subsequently, small sediment particles were removed with a fine needle under the binocular microscope. Altogether, 122 individual eggs were cleaned this way. Ten individual eggs were randomly selected to produce several-micron-thick slices (only five slices were finally available for further analysis). The slices were first observed under the optical microscope, and then uncoated slices were analyzed using energy dispersive spectrometry (EDS) for selected point analysis of elemental compositions and for elemental maps. The other 112 individual eggs were coated with gold and observed using the SEM. All referred specimens are deposited in the Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences, China (three slabs of eggs with carapaces of E. elliptica NIGP 161062-161064; five slices NIGP 161065-161069; 112 individual eggs NIGP 161070-161181).
Results
All the eggs are isolated from each other, and their distribution is relatively irregular, showing, apart from clustering, no trace of orientation (Fig. 1). Thirty-nine eggs that were affected by minor compaction but lack cracks or plastic deformation (see discussion below) were measured. The diameter of the eggs ranges from 126 μm to 172 μm, with an average of approximately 149 μm (Fig. 2.1). The number of the eggs in each specimen varies between 70 and 200. The length of the valve and the total number of the eggs (including the impressions left by eggs) were calculated for eight specimens. There appears to be a positive correlation between the size of the carapaces and the number of the eggs, although the dataset is admittedly too small for a statistically valid conclusion (Fig. 2.2).

Figure 2 Measurements of eggs of E. elliptica Chen, 1976. (1) Diameter of eggs that have suffered only minor compaction; (2) correlation between length of the carapace and total number of eggs (including impressions left by the eggs) based on eight specimens.
Some of the eggs had obviously experienced compaction because fragile cracks are observed (Fig. 3.7, 3.8). Excluding compactional deformation, the eggs come in two kinds of morphological varieties: well-rounded spheres (Fig. 3.1, 3.2) and spheres showing various indentations (Fig. 3.3–3.7, 3.9, 3.10, 3.16). The number of single dent vs. two dents vs. multi-dents (three or more) vs. well-rounded specimens is 20:15:35:34.The outer surface of the eggs is rather smooth (Fig. 3). In some of the eggs, there were very faint tracks, which differ from cracks caused by compaction (for compaction cracks see Fig. 3.7, 3.8; for regular faint tracks see Figs. 1.4, 3.13–3.16, 4.4). The length of these faint tracks, which taper toward both ends, is very uniform and approximately 10 μm (Fig. 4.4). They occur in both well-rounded and various indented specimens. Occasionally, they even form a ring around the egg surface, but we never found it around the equator line (Fig. 3.14–3.16, indicated by arrows).

Figure 3 SEM images of the three-dimensionally preserved eggs of Eosestheria elliptica Chen, 1976. (1, 2) Spherical specimens covered with the fossilized tertiary envelope, showing the smooth outer surface, NIGP 161070-161071; (3–6) specimens with various indentations, NIGP 161072-161075; (7) a specimen with indentations, slightly affected by compaction, NIGP 161076; (8) a heavily compacted specimen, NIGP 161077; (9, 10) specimens without the tertiary envelope but with indentations, NIGP 161078-161079; (11, 12) remains of the wrinkled layer seen below the partially removed tertiary envelope, NIGP 161080-161081; (13–16) regular arranged faint tracks are seen in some specimens with a well-preserved tertiary envelope, NIGP 161082-161085, arrow indicated these faint tracks form a ring surrounding the egg surface.

Figure 4 (1–3) details of the wrinkled layer below the tertiary envelope, from specimens NIGP 161080, 161081, 161086; (4) details of the regular faint tracks on the surface of the envelope, from specimen NIGP 161087. All SEM images.
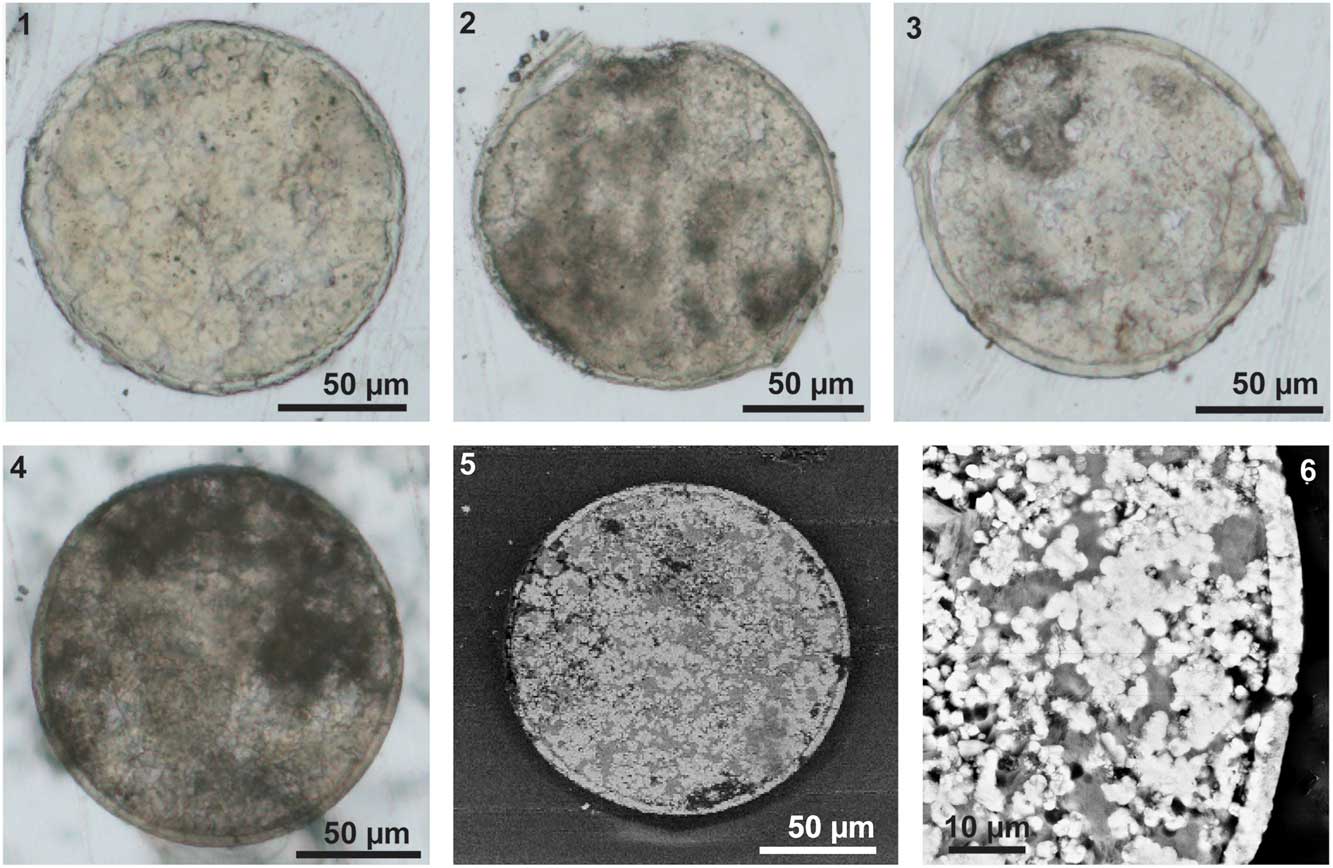
The egg has a relatively thick envelope, which is approximately 3–6 μm thick (Fig. 5), although in some cases part of the inner boundary of the envelope is not clear (Fig. 5.1). The thickness of the envelope is quite constant. In some broken specimens, there is a wrinkled layer below the envelope (Fig. 3.11, 3.12; Fig. 4.1–4.3). The wrinkled layer is much thinner, less than 1 μm thick (Fig. 4.1, 4.2; Fig.5.3). This layer is hardly detectable in the SEM images or elemental maps of the slices generated with EDS (Figs. 5.5, 5.6, 6). In some specimens, only the infilled part of the egg was observed, with the fossilized egg envelope being left behind when picked up from the sediment (Fig. 3.9, 3.10). These internal contents also show various indentations (Fig. 3.9, 3.10).
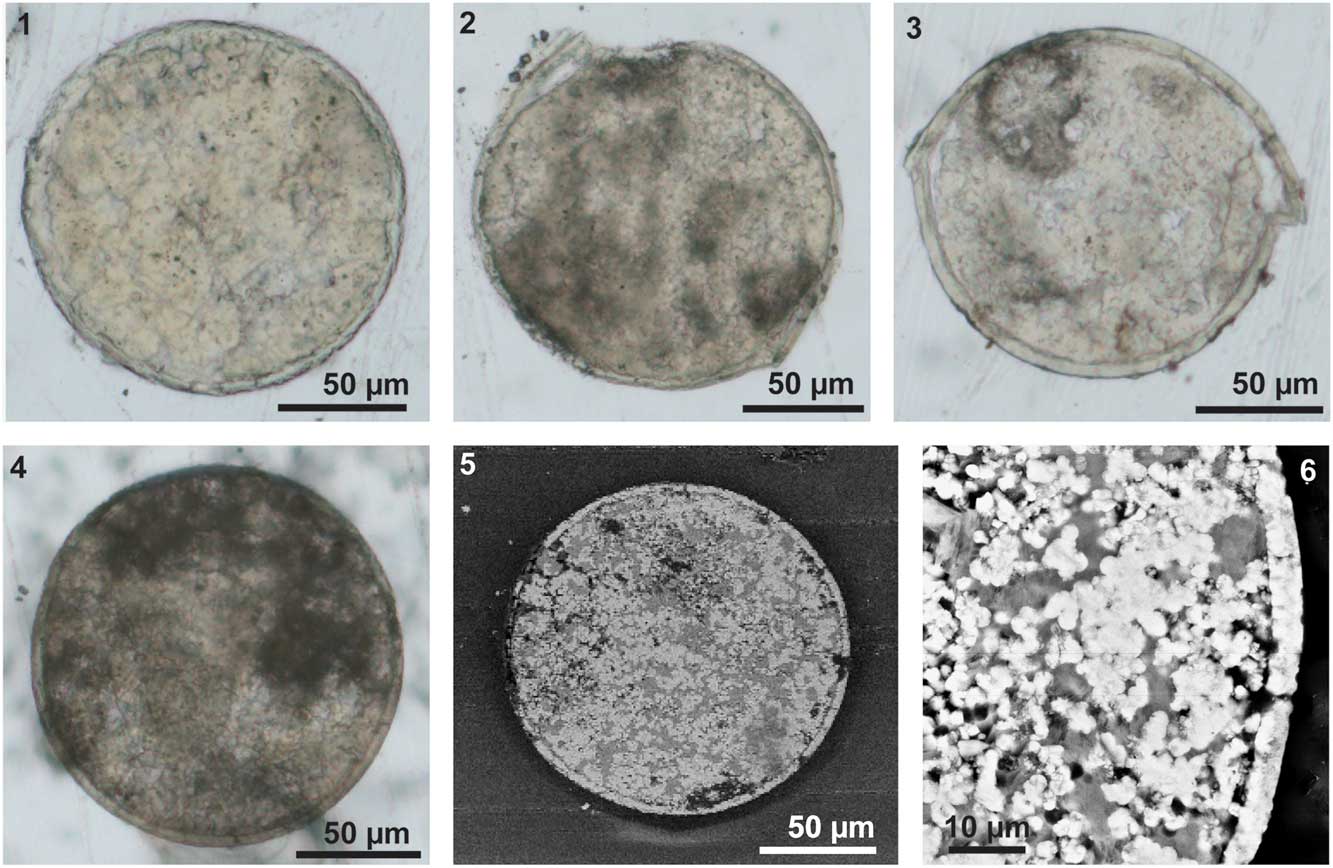
Figure 5 Slices of the three-dimensional eggs of Eosestheria elliptica Chen, 1976. (1–4) Images of slices, taken under an optical microscope, NIGP 161065-161068; (5, 6) SEM backscatter images of slice NIGP 156317 (same as Fig. 4.4), uncoated.

Figure 6 Results of energy X-ray spectrometer (EDS) for selected points analysis of elemental compositions and elemental maps of the uncoated slice (NIGP 161068). (1) EDS analysis for P from the field of view shown in image Fig. 6.8; (2) EDS analysis for Ca from the field of view shown in image Fig. 6.8; (3) EDS analysis for O from the field of view shown in image Fig. 6.8; (4) EDS analysis for F from the field of view shown in image Fig. 6.8; (5) EDS analysis for Ai from the field of view shown in image Fig. 6.8; (6) EDS analysis for Si from the field of view shown in image Fig. 6.8; (7) EDS analysis for Na from the field of view shown in image Fig. 6.8.
EDS analyses of the fossilized envelope revealed a calcium phosphate composition with accessory amounts of Al, Mg, Na, and F (Fig. 6). The particles filling the eggs display two completely different elemental compositions. Some exhibit the same elemental composition as the envelope (Fig. 6), whereas others have been replaced by alumino-silicate (Fig. 6).
Discussion
According to the life span of modern clam shrimps, usually a very short interval of time is available for hatching, and the eggs are evidently capable of remaining viable even under extremely harsh climatic conditions, such as desiccation or freezing (Tasch, Reference Tasch1984, Reference Tasch1987; Babcock et al., Reference Babcock, Isbell, Miller and Hasiotis2002; Clegg, Reference Clegg2005). Under these extreme conditions, the eggs are surrounded by a thick so-called tertiary envelope (Spotte and Anderson, Reference Spotte and Anderson1988). The size of clam shrimp eggs varies between 100 and 250 μm in diameter (Dumont and Negrea, Reference Dumont and Negrea2002, p. 134). For example, Eoleptesheria ticinensis and Cyzicus tetracerus have smooth and spherical eggs with diameters of approximately 125–130 μm and 145–150 μm, respectively (Thiéry and Gasc, Reference Thiéry and Gasc1991). The recorded fossil eggs of clam shrimps are usually approximately 100–150 μm in diameter, e.g. Estheria middendorfii 110–140 μm (Jones, Reference Jones1862), Estheria minuta approximately 130 μm (Grauvogel, Reference Grauvogel1947), Palaeolimnadia alsatica 100–130 μm (Tintori, Reference Tintori1990), Euestheria luanpingensis approximately 130 μm (Shen and Huang, Reference Shen and Huang2008), and Montcestheria orri 135-144 μm (Vannier et al., Reference Vannier, Thiéry and Racheboeuf2003). There are two exceptions: Pruvost (Reference Pruvost1919) recorded extremely large eggs of Estheria striata with diameters up to 580 μm, and Dechaseaux (Reference Dechaseaux1951) recorded much smaller eggs of Palaoelimnadia sp. with diameters ranging from 20 to 75 μm. Comparable size ranges were obtained for the fossil eggs of E. elliptica with an average diameter of 149 μm (Fig. 2.1). Compared with their extant relatives, the eggs of Lower Cretaceous clam shrimps display a greater size variation and a slightly thinner envelope (see below). The fossil eggs were found within the carapace, suggesting they were still attached to the body before they were buried. The polymolecular complex forming the egg envelops might have experienced dehydration to various degrees before being finally fossilized, which would affect the size of the fossils.
The fossil eggs are surrounded by a uniform and relatively thick layer (3–6 μm thick), which is most likely corresponding to the tertiary envelope (Fig. 5). The thickness of the tertiary envelope in extant groups is approximately 4.5%–5% of the diameter (Thiéry and Gasc, Reference Thiéry and Gasc1991), so the fossil tertiary envelope is slightly thinner than that of extant ones. In extant clam shrimp eggs, the tertiary envelope is composed of an outer membrane, a cortical layer, and a thick alveolar layer, which is composed of lipoprotein complexes (Morris and Afzelius, Reference Morris and Afzelius1967; Gilchrist, Reference Gilchrist1978; Munuswamy and Subramoniam, Reference Munuswamy and Subramoniam1984; Spotte and Anderson, Reference Spotte and Anderson1988; Tommasini and Sabelli, Reference Tommasini and Sabelli1989; De Walsche et al., Reference De Walsche, Munuswamy and Dumont1991; Lee et al., Reference Lee, Gouthro, Belk and Rosowski1994; Rosowski et al., Reference Rosowski, Belk, Gouthro and Lee1997). These layers have different preservational capacities, and probably only the thick alveolar layer can be mineralized and preserved. Below the thick tertiary envelope, there is a thin wrinkled layer (Fig. 4.1–4.3). A similar wrinkled layer of the first embryonic cuticle has been reported on extant clam shrimp eggs (Spotte and Anderson, Reference Spotte and Anderson1988; Rosowski et al., Reference Rosowski, Belk, Gouthro and Lee1997). Thus the wrinkled layer is assumed to be the first cuticle of the embryo.
The palaeolake system was characterized by dysoxic bottom waters with spells of anoxia (Fürsich et al., Reference Fürsich, Sha, Jiang and Pan2007; Pan et al., Reference Pan, Sha, Fürsich, Wang, Zhang and Yao2012; Hethke et al., Reference Hethke, Fürsich, Jiang and Pan2013). Thanks to their thickly enveloped cysts, the clam shrimps could repeatedly populate the lake. Fragile cracks have been found in several specimens (Fig. 3.7, 3.8). Experiments suggest that the force required to crush branchiopod cysts is very small, for example less than 1 newton is required for dry cysts, while wet cysts are even more fragile (Hathaway et al., Reference Hathaway, Sheehan and Simovich1996). So such fragile cracks could happen during the burial process or later compaction. The indentation morphostructures (Fig. 3.3–3.7, 3.9, 3.10, 3.16) show the flexibility instead of fragility of the eggs. Hill and Shepard (Reference Hill and Shepard1997) studied the eggs of modern Artemia franciscana, and found similar indentations, which were explained as the embryo not filling the entire volume circumscribed. Munuswamy and Subramoniam (Reference Munuswamy and Subramoniam1984, fig. 2) illustrated that the embryo is segregated to one side with no material on the other, which has also been confirmed by Belk (Reference Belk1987, fig. 5). Liu et al. (Reference Liu, Zhao and Dai2009, fig. 1b-a’) implied that a singular dent could also represent the blastopore. However, in our case, one dent specimens occupies less than one-fifth of the studied eggs (20 vs. 104), and occupies two-sevenths of the studied indentation eggs (20 vs. 70). These explanations may be applicable to one dent specimens, but not to the well-rounded specimens or two- and multi-dent specimens, which all occur in reasonable numbers. A combined influence of post-burial compression, dehydration, and early mineralization is assumed to account for indentations of the fossil eggs.
Another interesting phenomenon observed is that the eggs cluster in the middle of the carapace (Fig. 1.1, 1.2). Zhang et al. (Reference Zhang, Shen and Niu1987) proposed that the eggs of Euestheria luanpingensis, situated in a similar position, were attached to the exopod of the trunk limbs. All extant Spinicaudata have eggs/embryos attached to the dorsal prolongation of the exopod, and Olesen (Reference Olesen1998) thought that the attachment of eggs to the exopod is an evolutionary intermediate between ventrally situated eggs and dorsally situated eggs. This finding confirms that attachment of the eggs to the exopod is a plesiomorphic feature already present in Mesozoic Spinicaudata.
The results of EDS analyses and elemental maps suggest that calcium phosphate comprises the outer wall of the egg and some particles of the filling (Figs. 4 and 5). The tertiary envelope is originally composed of lipoprotein complexes (Spott and Anderson, Reference Spotte and Anderson1988), and the embryo within the envelope is non-biomineralized and composed of polymolecular complexes. Some eggs of crustaceans harden after they are shed from the female to the substrate (Browe et al., Reference Browe, Price, Gerberding and Patel2005), which means calcium phosphate might be precipitated within the framework of the lipoprotein complexes of the tertiary envelope, as in some cases of the carapace valves (Dudich, Reference Dudich1931; Tasch, Reference Tasch1969; Rieder et al., Reference Rieder, Abaffy, Hauf, Lindel and Weishäupl1984; Stigall et al., Reference Stigall, Babcock, Briggs and Leslie2008). Up to now, there is only a single report of true soft-part preservation (e.g. appendages) of clam shrimps from the Jehol Biota (Shen, 2011, appendages of Eosestheria lingyuanensis Chen, 1976 from the Yixian Formation), but we have failed to recognize any such soft parts in our collection. The abundant carapace valves of the clam shrimps are replaced by recrystallized fluorapatite (Hethke et al., Reference Hethke, Fürsich, Jiang and Pan2013). Thus, we suggest that the tertiary envelope of the eggs and carapaces of the clam shrimp possibly share some common microstructures that are responsible for their similar preservation. For example, the hollow space within their framework may allow minerals to grow in situ at the very early stages of decay. More work is certainly needed to determine which mechanisms were responsible for the preservational pattern of clam shrimps from the Yixian Formation.
In addition to early mineralization of the tertiary envelop, early mineralization of the hollow space left by the decay of the embryo must have occurred to prevent the collapse of the eggs during compaction. Otherwise, their preservation as three-dimensional fossil structures would not have been possible (Briggs, Reference Briggs2003; Martin et al., Reference Martin, Briggs and Parkes2003; Orr et al., Reference Orr, Briggs and Kearns2008). We hypothesize that the calcium phosphate first replaced the framework of the tertiary envelope. The carapaces themselves could well be the primary sources of the phosphate, although it might have been derived from other sources as well. Phosphatization of the void left by decay of the embryo is very likely facilitated by the tertiary envelope, which provided a microenvironment with a higher content of phosphate from the decaying embryo. It is notable that alumino-silicification also happened extensively, as some of the particles within the egg had been replaced by alumino-silicate (Fig. 5).
Conclusions
The fossil eggs of E. elliptica Chen, 1976 are characterized by a spherical shape with smooth surface. The fossilized tertiary envelope consists of calcium phosphate and is approximately 3–6 μm thick. The first embryonic cuticle with wrinkled structures on the outer surface is still preserved. Probably the tertiary envelope provided an isolated microenvironment with a higher phosphate content, which favored early mineralization within the eggs. Moreover, this study also adds new information on the exceptional preservation of the Jehol Biota.
Acknowledgments
We are grateful to Dr. T.A. Hegna, Prof. Z.H. Zhou, and Dr. J. Haug for useful information, critical review, and improvement of the manuscript; X.L. Zhang for helping to collect the specimens; F. T. Fürsich, D.Y. Huang, and C.M. Zhou for discussions; Y. Fang and Y.Q. Mao for technical assistance on the scanning electron microscope and X-ray spectrometer; and L.F. Gao for making the slices. The study was supported by the National Natural Science Foundation of China (91114201, 41102005), and the National Basic Research Program of China (973 program; 2012CB821906).