Introduction
An Amphoracrinus Austin, Reference Austin1848 specimen from England was the first illustrated Paleozoic crinoid (Lister, Reference Lister1673; Ausich and Kammer, Reference Ausich and Kammer2006). Amphoracrinus is now known from Western Europe, the United States, and China (Tables 1 and 2). The genus had a duration of as much as 20 million years from the Famennian through the early Viséan (Mississippian). With the addition of Amphoracrinus tenax new species (Muldraugh Member, Borden Formation, early Viséan), 17 named species (one with a named variation) are currently assigned to this genus (Wright, Reference Wright1943, Reference Wright1955; Chen and Yao, Reference Chen and Yao1993; Ausich and Sevastopulo, Reference Ausich and Sevastopulo2001; Donovan et al., Reference Donovan, Lewis and Kabrna2006; Ausich and Kammer, Reference Ausich and Kammer2006, Reference Ausich and Kammer2008a; Webster et al., Reference Webster, Waters and Chen2009) (Table 1).
Table 1. Species of Amphoracrinus listed by country occurrence with appropriate international ages as well as regional stages.

Table 2. Chronostratigraphy of the Mississippian. Correlation of International series, European and North American regional stages, and time bins of Ausich and Kammer (Reference Ausich and Kammer2006) and Kammer and Ausich (Reference Kammer and Ausich2007).

Despite its relatively short temporal duration, Amphoracrinus spread across much of the globe in the northern tropics and subtropics, in both western and eastern Laurussia and on the Sibumasu Block, which was part of northern Gondwana during the Mississippian (present-day southern China). The most common occurrences of Amphoracrinus are on the Tournaisian mixed carbonate and siliciclastic ramp of southern Ireland and southwestern United Kingdom (Hook Head Formation, Ausich and Sevastopulo, Reference Ausich and Sevastopulo1994, Reference Ausich and Sevastopulo2001) and in facies associated with Tournaisian and Viséan Waulsortian buildups in Ireland, England, and Wales (Wright, Reference Wright1955).
The Mississippian, recognized as the “Age of Crinoids” (Kammer and Ausich, Reference Kammer and Ausich2006; Ausich et al., Reference Ausich, Kammer, Mirantsev, Lucas, Schneider, Wang and Nikolaeva2020a), is known for diverse and abundant crinoid occurrences, yet Amphoracrinus is absent from all but four North American crinoid faunas. Surprisingly, with the description of A. tenax n. sp. (Fig. 1), both the oldest named species, A. viminalis (Hall, Reference Hall1863) (Meadville Shale Member, Cuyahoga Formation, early Tournaisian, Kinderhookian, late Hastarian of Ohio), and the youngest species, A. tenax n. sp. (Muldraugh Member, Borden Formation, early Viséan, late Osagean, Arundian of Kentucky), occur in the United States. The taphonomic implications of A. tenax are also discussed in this study

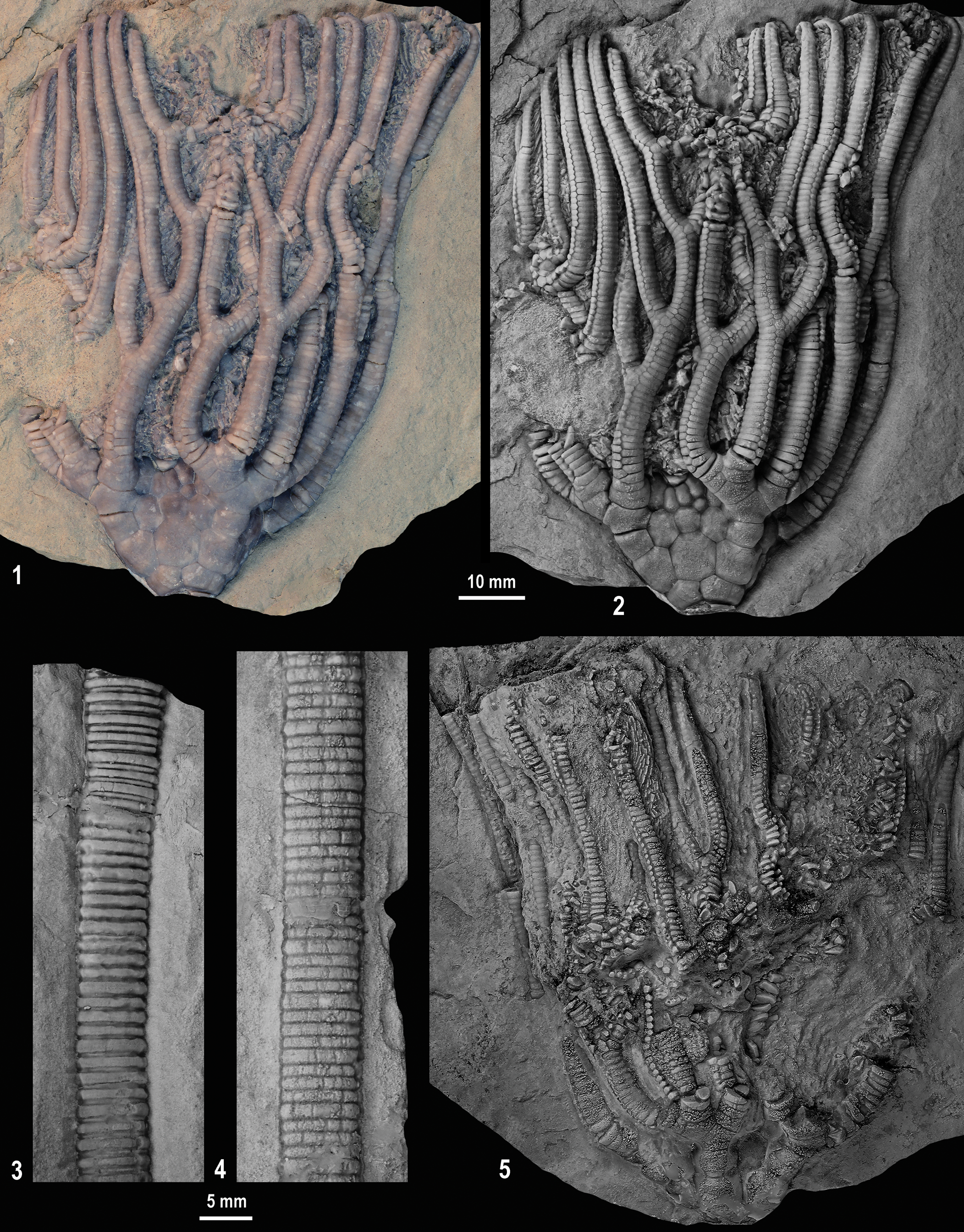
Figure 1. Amphoracrinus tenax n. sp. holotype from the Muldraugh Member (early Viséan) of the Borden Formation, north-central Kentucky. Posterior side of entire specimen. USNM PAL 771320, holotype.
Location and stratigraphy
This new specimen was collected from the Muldraugh Member of the Borden Formation in north-central Kentucky. This location differs from the Muldraugh location (a quarry) described previously by Ausich et al. (Reference Ausich, Goldstein and Yates2000) and Kammer et al. (Reference Kammer, Ausich and Goldstein2007). The specimen was collected from a road cut on Kentucky route 313 in Hardin County, approximately 7.3 km west of I-65 (exit 102), coordinates 37°48′23.492″N, 85°49′29.513″W (UTM 16N 591623 4184859) (Fig. 2). The road-cut stratigraphy includes (in ascending order) the New Providence Shale Member of the Borden Formation, the Muldraugh Member of the Borden Formation, and the Harrodsburg Limestone (Kepferle, Reference Kepferle1977) that resulted from the time-transgressive, basin-filling progradation of the Borden Delta (e.g., Lane and DuBar, Reference Lane and DuBar1983; Richardson and Ausich, Reference Richardson and Ausich2004). The Muldraugh Member has much lenticular bedding (Ausich et al., Reference Ausich, Goldstein and Yates2000), and this new specimen was collected from loose matrix on the surface of a lenticular bed approximately 28 m above the New Providence Shale Member (Fig. 2).

Figure 2. Road cut in Hardin County, north-central Kentucky, where A. tenax n. sp. was collected from the Muldraugh Member of the Borden Formation. Lithostratigraphy noted on right; arrow indicates its location on the bench.
The Muldraugh Member was a mixed carbonate-siliciclastic, tempestite-dominated ramp environment and the shallow-water equivalent of the Fort Payne Formation in Kentucky and Tennessee (Nicoll and Rexroad, Reference Nicoll and Rexroad1975; Peterson and Kepferle, Reference Peterson and Kepferle1970; Kammer and Ausich, Reference Kammer and Ausich1987; Ausich and Meyer, Reference Ausich and Meyer1990; Sable and Dever, Reference Sable and Dever1990; Ausich et al., Reference Ausich, Goldstein and Yates2000). The Muldraugh Member consists of argillaceous, coarse-grained packstones, yellow-gray dolomitic siltstone, silty dolomite, crinoidal limestone, shale, chert, and geodes (Sable et al., Reference Sable, Kepferle and Peterson1966; Kepferle, Reference Kepferle1977; Sable and Dever, Reference Sable and Dever1990; Ausich et al., Reference Ausich, Goldstein and Yates2000). In the Muldraugh Member, advanced cladids, followed by camerates, dominated in both species richness and the number of specimens (Ausich et al., Reference Ausich, Goldstein and Yates2000). Other contemporaneous deltaic localities with a comparably diverse crinoid fauna include the New Providence Shale Member (prodeltaic environments) in southern Indiana and north-central Kentucky (Ausich et al., Reference Ausich, Kammer and Lane1979; Kammer, Reference Kammer1984), the Edwardsville Formation (delta platform environments) in central and southern Indiana (Lane, Reference Lane1963, Reference Lane1973; Van Sant and Lane, Reference Van Sant and Lane1964; Ausich and Lane, Reference Ausich and Lane1982), and the Fort Payne Formation of south-central Kentucky (Krivicich et al., Reference Krivicich, Ausich and Meyer2014 and references cited therein). In other locations, the Muldraugh Member contains a diverse assemblage, including brachiopods, bryozoans, corals (primarily rugosans), conulariids, other echinoderms, gastropods (abundant platyceratids), sponges (abundant hexactinellids), arthropods, trace fossils, and vertebrates (Chondrichthyes teeth and dorsal fin spines). However, in the bed containing A. tenax, the only other skeletal elements are crinoid columnals and pluricolumnals.
Methods
Table 1 is a list of taxa considered in this study, including their distribution and age. Note that Ausich and Kammer (Reference Ausich and Kammer2008a) designated A. blairi Miller and Gurley, Reference Miller and Gurley1896a and A. jessieae Miller and Gurley, Reference Miller and Gurley1896b as nomen dubia; Amphoracrinus rouchi Delpey, Reference Delpey1941 was transferred to Ectocrinus by Webster et al. (Reference Webster, Maples, Sevastopulo, Frest and Waters2004); and A. atlas (M'Coy, Reference M'Coy1849), A. bollandensis Wright, Reference Wright1955, and A. gilbertsoni (Miller in Phillips, Reference Phillips1836) are only questionably recognized in China (Webster et al., Reference Webster, Waters and Chen2009). In addition, Webster and Waters (Reference Webster, Waters and Königshof2009) described two poorly preserved Devonian crinoids from northwestern China as Amphoracrinus sp. These specimens are from the Hongguleleng Formation (Famennian). Unfortunately, both specimens are too poorly preserved to code their morphological characters.
Cluster analyses were conducted using PAST 4.04 (Hammer and Harper, Reference Hammer and Harper2006; Hammer et al., Reference Hammer, Harper and Ryan2006). The paired group (UPGMA) algorithm, the Euclidean similarity index, and the no constraints setting were employed to generate dendrograms using the characters listed in Supplemental Tables 1, 2.
Parsimony analyses were performed using PAUP 4.0a168 (Swofford, Reference Swofford2015). Analyses were run using the criterion of maximum parsimony and heuristic searches with random addition repeated 1,000 times. All characters were treated as equally weighted and unordered. Branch swapping was with the tree bisection–reconnection algorithm. Strict consensus, 50% majority rule, and Adams consensus trees were evaluated, the latter to identify potential “wildcard taxa” for possible elimination (Wiley and Lieberman, Reference Wiley and Lieberman2011). The consistency index (ci), retention index (ri), and rescaled index (rc) were calculated, and new trees were generated using rescaled consistency indices. Bayesian, tip-dating method was not attempted because there is insufficient temporal resolution for this approach. Combined, the outgroup taxa span much of the Devonian, but all ingroup species belong to either one or both of two time bins (Tournaisian and Viséan). Tree support was evaluated with bootstrap values, jackknife values, and Bremer support.
Three outgroup taxa are designated. All are from the Periechocrinidae, which were probably ancestors of the Amphoracrinidae (Ausich and Kammer, Reference Ausich and Kammer2008a, Reference Ausich, Kammer, Ausich and Webster2008b). Outgroup taxa are Athabascacrinus orientale (Waters et al., Reference Waters, Maples, Lane, Marcus, Liao, Liu, Hou and Wang2003) (Famennian, China), Pyxidocrinus collensis Breimer, Reference Breimer1962 (Emsian, Spain), and Megistocrinus depressus (Hall, Reference Hall1862) (Eifelian-Givetian, United States). Numerous morphological characters were coded; however, based on results of PAUP, only 16 were informative for 14 species of Amphoracrinus and three outgroup taxa (Table 1, Supplementary Tables 2, 3). The initial parsimony analysis yielded 1,460 trees with a length of 55. An Adams consensus tree yielded more than one polytomy, and the following five wildcard species were identified and eliminated from further analyses: A. atlas, A. bollandensis, A. portlocki Wright, Reference Wright1955, A. rotundus Wright, Reference Wright1955, and A. turgidus Wright, Reference Wright1943.
With these wildcard taxa eliminated, the resulting analysis yielded 91 trees of length 45. However, as noted, this tree lacked any bootstrap, jackknife, or Bremer support. Next, trees were generated using rescaled consistency indices (rc), resulting in 12 trees of length 26.64722 (Fig. 3.2) that also lacked any bootstrap, jackknife, or Bremer support.
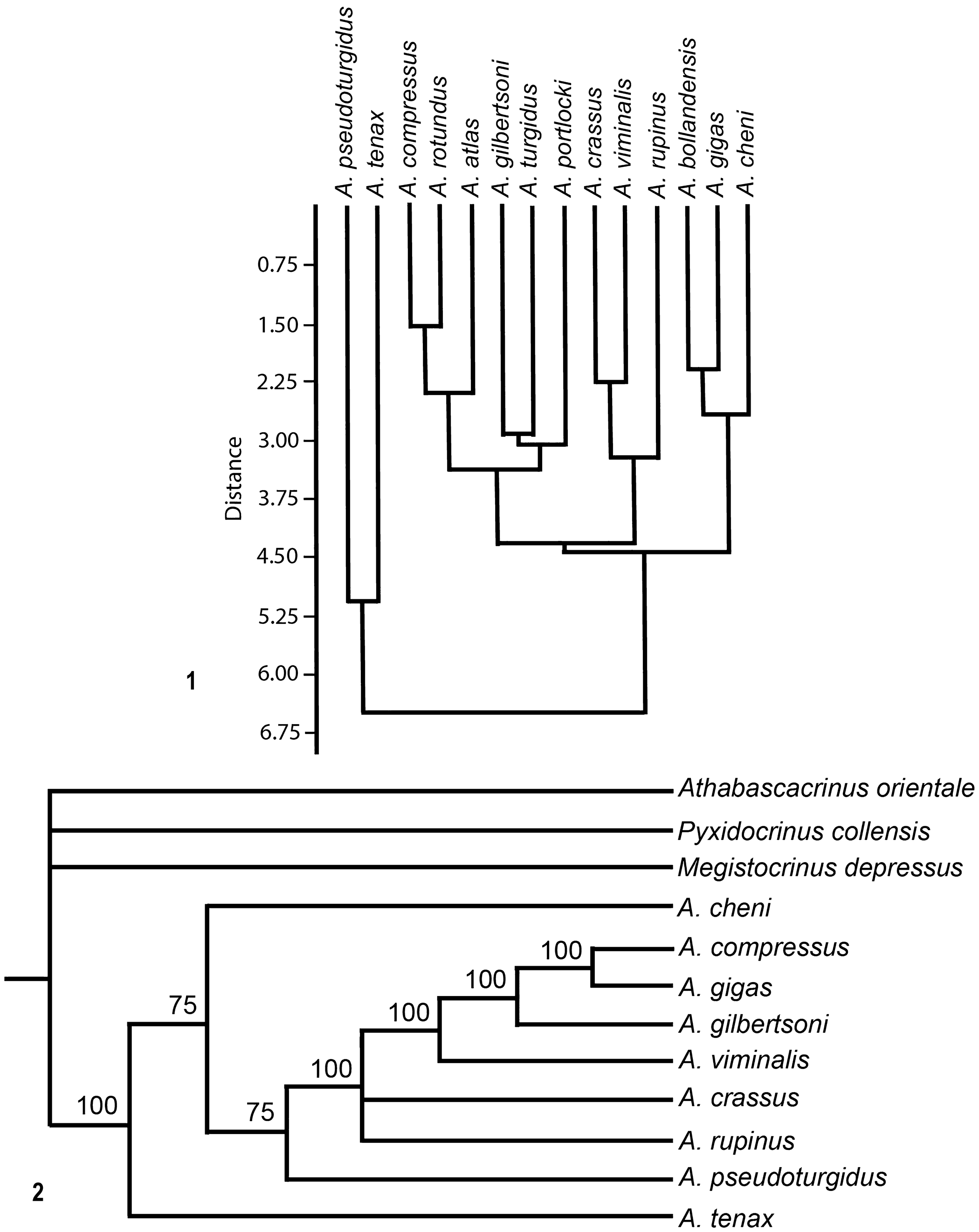
Figure 3. Results from morphological analyses of Amphoracrinus. (1) Dendrogram from cluster analysis of morphological characters of Amphoracrinus species. (2) A 50% majority-rule tree of nine species of Amphoracrinus with three outgroup taxa. Values at nodes indicate the percentage of most parsimonious trees that recovered that relationship (data in Supplemental Tables 1, 2, 3).
Repository and institutional abbreviation
The holotype of A. tenax n. sp. is deposited in the Smithsonian National Museum of Natural History (USNM), USNM PAL 771320.
Systematic paleontology
The superfamilial classification used here follows Cole (Reference Cole2017), Wright (Reference Wright2017), and Wright et al. (Reference Wright, Ausich, Cole, Peter and Rhenberg2017); family-level classifications follow Moore and Teichert (Reference Moore and Teichert1978). Morphologic terminology follows Webster (Reference Webster1974), Ubaghs (Reference Ubaghs, Moore and Teichert1978b), and Ausich et al. (Reference Ausich, Wright, Cole and Sevastopulo2020b). The plating of interrays is given by the number of plates in each range, from proximalmost plate to the last range before the tegmen. In the posterior interray, the primanal is indicated by “P,” and the first interradial plate in regular interrays is indicated by “1.” A “?” indicates that more-distal plating is unknown (Ausich, Reference Ausich and Ausich2021a). Abbreviations used in designating measurements include the following: CrH, crown height; CaH, calyx height; CaW, calyx width; CoH, column height. An * indicates a measurement was incomplete.
Class Crinoidea Miller, Reference Miller1821
Subclass Camerata Wachsmuth and Springer, Reference Wachsmuth and Springer1885
Infraclass Eucamerata Cole, Reference Delpey2017
Order Monobathrida Moore and Laudon, Reference Moore and Laudon1943
Suborder Composocrinina Ubaghs, Reference Ubaghs, Moore and Teichert1978a
Superfamily Periechocinacea Bronn, Reference Bronn1849
Family Amphoracrinidae Bather, Reference Bather1899
Genus Amphoracrinus Austin, Reference Austin1848
Type species
Amphoracrinus gilbertsoni Miller in Phillips, Reference Phillips1836.
Included species
A. atlas (M'Coy, Reference M'Coy1849); A. bollandensis Wright, Reference Wright1955; A. cheni Webster et al., Reference Webster, Waters and Chen2009; A. compressus Wright, Reference Wright1943; A. crassus (Austin and Austin, Reference Austin and Austin1843); A. gigas Austin in Wright, Reference Wright1955; A. gilbertsoni (Miller in Phillips, Reference Phillips1836); A. granulatus (Austin and Austin, Reference Austin and Austin1843); A. portlocki Wright, Reference Wright1955; A. pseudoturgidus Chen and Yao, Reference Chen and Yao1993; A. rotundus Wright, Reference Wright1955; A. rugosus (Rofe, Reference Rofe1865); A. rupinus Webster and Lane, Reference Webster and Lane1987; A. tenax n. sp.; A. turgidus Wright, Reference Wright1943; and A. viminalis (Hall, Reference Hall1863).
Type
The holotype of A. tenax n. sp. is designated USNM PAL 771320.
Diagnosis
Calyx low cone shape; calyx plates convex, smooth; basal circlet 20% of calyx height, ridge at base of calyx absent; radial plates as high as wide, unequal in size, smaller than primanal; three interradial plates in regular interrays with distalmost range adjacent to primibrachials, regular interrays depressed; first fixed primibrachial tetragonal, as high as wide; CD interray very much wider than other regular interrays, three plates above primanal; first primibrachial smaller than radial plate and primaxil, more than five CD-interray plates in calyx; two fixed primibrachials, 15–20 free arms project upward and branch.
Occurrence
Muldraugh Member of the Borden Formation, Hardin County, north-central Kentucky, 37°48′23.492″N, 85°49′29.513″W, UTM 16N 591623 4184859.
Description
Calyx medium sized, low cone shape (Fig. 4.1), ~0.52 times higher than wide; arms grouped and interrays moderately depressed; calyx plates low convex shape, smooth plate sculpturing. Basal circlet high, visible in side view, ~20% of calyx height (Fig. 4.2); three basal plates, unequal in size, wider than high, much smaller than radial plates. Radial circlet ~35% of calyx height; radial plates five, hexagonal, as high as wide. Regular interrays narrowly in contact with tegmen, all plates convex as noted; first interradial hexagonal, as high as wide, smaller than radials and first primibrachial; plating in second range unknown. Primanal heptagonal, higher than wide, largest plate in calyx, interrupts the radial circlet; plating in CD interray P–3–5–? (Fig. 4.2), plates have impressed sutures; second range of interradial plates in posterior with subtle plate plications that are ridges that connect with like ridges on adjoining plates (radials, primanal, first and second primibrachials, adjacent plates in the first and second ranges of interradial plates, and some plates of the third range of posterior interray plates) (Fig. 4.2). CD interray in contact with tegmen. First primibrachial tetragonal or pentagonal, approximately as high as wide, convex, smaller than radial plates and primaxil; second primibrachial axillary, approximately twice as wide as high. Intrabrachial plates between adjacent half-rays absent. Tegmen not visible.
Three or four free arms per ray (C ray four arms; A and D rays three arms). Free arms begin with first tertibrachial, first tertibrachial either uniserial or biserial (Fig. 4.2); all subsequent brachials biserial. Free arms robust, aborally rounded; directed obliquely upward, narrow distally, and abaxially incurved at distal end of crown. Each free arm may have as many as four branches (Fig. 4.2), with branching pattern variable above the first branch (may be either endotomous or exotomous) (Fig. 4. 2). Pinnules slender. Column circular (Figs. 1, 4); in proximal column heteromorphic (N212 pattern), latus of nodals very convex and latus of internodals progressively less convex (Fig. 4.3); mesistele with very subtle N212 pattern and some places approach homeomorphy, latus of columnals convex (Fig. 4.4).
Etymology
The name, tenax (m), is Latin for steadfast or tenacious.
Measurements
CrH, 90.0; CaH, 25.0*; CaW, 12.0; CoH, 230*.
Remarks
USNM PAL 771320 is the most complete known specimen of this genus. Most species are known from only the theca (calyx and tegmen). Because the arms are largely complete, they hide the tegmen, so comparison to other species is limited to characters of the calyx.
Amphoracrinus tenax n. sp. differs from other North American species because A. rupinus has a very low cone shape, coarsely granulose plate sculpturing, a continuous ridge around the base of the calyx, radial plates wider than high and the largest plates in the calyx, most-distal plates in calyx interrays that abut secundibrachials, first primibrachial wider than high, CD interray wider than other interrays, three or four extra plates in the CD interray, and 10 free arms. By contrast, A. viminalis has a very low bowl-shaped calyx, coarsely granulose plate sculpturing, a broken ridge around the base of the calyx, radial plates wider than high and the largest plates in the calyx, distal-most interradials in calyx that abut secundibrachials, first primibrachial wider than high, CD interray wider than other interrays, two plates above the primanal, and 10 free arms (see Supplemental Table 5 for comparison with other species).
Results and discussion
Paleogeography and evolution
Amphoracrinus viminalis, the oldest named species, is from northeastern Ohio from the Meadville Shale Member of the Cuyahoga Formation and dates to the late Kinderhookian (Tournaisian, late Hastarian) (Table 1). The Meadville Shale was deposited in a shallow marine deltaic tempestite shelf setting that resulted in excellent crinoid preservation (Ausich and Roeser, Reference Ausich and Roeser2012; Kammer and Roeser, Reference Kammer and Roeser2012). A. rupinus (Webster and Lane, Reference Webster and Lane1987) is from southern Nevada (Meadow Valley Range) from the Anchor Limestone of the Monte Cristo Group (early Osagean, Tournaisian, Ivorian), where monobathrid camerates were the dominant element of the crinoid fauna (Webster and Lane, Reference Webster and Lane1987). A. rupinus was also documented in New Mexico from the Nunn Member of the Lake Valley Formation (early Osagean/late Tournaisian) (Rhenberg and Kammer, Reference Rhenberg and Kammer2013). The Nunn Member in general was a narrow and relatively deepwater low-energy carbonate ramp with Waulsortian mounds; however, the specific location where Amphoracrinus occurred was from a shallower shelf subenvironment comparable to the Burlington Shelf (Rhenberg and Kammer, Reference Rhenberg and Kammer2013). Amphoracrinus tenax n. sp. is now the youngest (early Viséan, Arundian) described species of Amphoracrinus.
Amphoracrinus is best known from Western Europe, where it occurs from the Tournaisian (Ivorian) to the early Viséan (upper Chadian) in England, Wales, Ireland, and Belgium. In Ireland, Amphoracrinus is present in the Hook Head Formation (Tournaisian, Ivorian), particularly the shallow-water “Michelinia Beds” (Ausich and Sevastopulo, Reference Ausich and Sevastopulo2001), which was a mixed carbonate and siliciclastic tempestite shelf-ramp (Ausich and Sevastopulo, Reference Ausich and Sevastopulo1994). It is also present in the equivalent ramp setting in southwestern United Kingdom. Amphoracrinus was a dominant faunal element in the Tournaisian (lower Chadian) to the Viséan (upper Chadian) Waulsortian-related facies in England, Wales, Ireland, and Belgium (Wright, Reference Wright1955; Ausich and Sevastopulo, Reference Ausich and Sevastopulo1994, Reference Ausich and Sevastopulo2001; Ausich and Kammer, Reference Ausich and Kammer2006, Reference Ausich and Kammer2008a; Donovan et al., Reference Donovan, Lewis and Kabrna2006).
As noted, the oldest occurrence of Amphoracrinus is from the Famennian of China (Webster and Waters, Reference Webster, Waters and Königshof2009). Chen and Yao (Reference Chen and Yao1993) described A. atlas, A. bollandensis, A. gigus, A. gilbertsoni, and A. pseudoturgidus from the Tournaisian of China. These species assignments were revisited by Webster et al. (Reference Webster, Waters and Chen2009), who described A. cheni and A. pseudoturgidus; but they recognized the occurrences A. atlas? and A. gilbertsoni? as questionable. Both the Famennian and Tournaisian occurrences in China are interpreted to have been from shallow-water habitats with no clear evidence of association with carbonate buildups.
Cluster analysis was performed on morphological characters (Supplemental Tables 1, 2) to identify any morphological similarities among Amphoracrinus species within and between geographic areas. In the resultant dendrogram, the three North American species are not grouped together. Amphoracrinus tenax n. sp. and A. pseudoturgidus form a group at the base of the dendrogram, and A. crassus, A. rupinus, and A. viminalis form a group (Fig. 3.1). Amphoracrinus bollandensis, A. gigas, and A. cheni group together, and the remaining Western European species (with A. rupinus and A. viminalis) group together in various clusters.
Phylogenetic analyses were also performed. Whereas the phylogenetic tree in Figure 3.2 cannot be demonstrated to be a robust phylogenetic solution, it displays interesting relationships, with European, North American, and Chinese species intermixed. For example, A. tenax, A. cheni, and A. pseudoturgidus (Tournaisian, China) are ladderized at the base of the ingroup (Fig. 3.2). By contrast, the two other North American species (A. viminalis, Tournaisian, and A. rupinus, Viséan) are in a clade with all European species. Amphoracrinus rupinus and A. crassus are in the crownward clade and are on a polytomy. A. viminalis is in a basal position of the crownward group. Thus, the three North American species are in reverse stratigraphic order and are associated with species of contrasting paleogeographies. Interpretation of the phylogenetic analysis (Fig. 3.2) is regarded as tentative, but the repeated recovery of relationships at the base of the tree suggest that these taxa have close phylogenetic and paleogeographic relationships. The older species of North American Amphoracrinus (A. rupinus and A. viminalis) are most closely associated with European species, whereas the youngest North American species (A. tenax n. sp.) is more closely associated with species from China, which may suggest either separate paleogeographic origins or multiple migration events for North American members of this genus. These relationships will not be resolved until characters of the tegmen, arms, and column are known for most species, which is unlikely. At least tegmen characters for A. tenax may become known with additional specimens, which is significant because tegmen characters are highly variable and phylogenetically important (Kammer et al., Reference Kammer, Sumrall, Zamora, Ausich and Deline2013; Ausich and Kammer, Reference Ausich and Kammer2016).
Ausich and Kammer (Reference Ausich and Kammer2013) evaluated the temporal and paleogeographic patterns among Mississippian crinoids in North America and Western Europe. On the basis of analyses of first occurrences of genera, paleobiogeography through the history of a genus, and biodiversity changes through the Mississippian, Ausich and Kammer (Reference Ausich and Kammer2013) concluded that the evolutionary center of monobathrid camerate crinoids, disparid crinoids, and cyathocrine crinoids was predominantly in North America, with subsequent migration to Western Europe. A major factor driving migration was interpreted to be sea-level rise following the late Kinderhookian glaciation (Walker et al., Reference Walker, Wilkinson and Ivany2002; Caputo et al., Reference Caputo, de Melo, Streel, Isbell, Fielding, Frank and Isbell2008; Kammer and Matchen, Reference Kammer, Matchen, Fielding, Frank and Isbell2008). Waters et al. (Reference Waters, Marcus, Maples, Lane, Hou, Liao, Wang, Liu, Ausich and Webster2008) and Webster et al. (Reference Webster, Waters and Chen2009) concluded that most Chinese Mississippian crinoids migrated from Western Europe. Thus, it follows that the Tournaisian crinoid faunas from China were originally seeded by migration from North America and Europe as a result of sea-level rise following late Kinderhookian glaciations.
With Amphoracrinus sp. (Webster and Waters, Reference Webster, Waters and Königshof2009) (Famennian) assigned to Amphoracrinus, a more-nuanced paleogeographic history must have existed. Origination of Amphoracrinus in northwestern China during the Famennian would be consistent with the overall conclusions of several studies that indicate that Famennian blastoids and crinoids in China have close affinities to early Mississippian crinoids in North America (Lane et al., Reference Lane, Waters and Maples1997; Waters et al., Reference Waters, Maples, Lane, Marcus, Liao, Liu, Hou and Wang2003, Reference Waters, Marcus, Maples, Lane, Hou, Liao, Wang, Liu, Ausich and Webster2008; Webster and Waters, Reference Webster, Waters and Königshof2009). This would suggest that during the latest Devonian and earliest Mississippian, migration routes were more freely open than traditionally understood between China, North America, and Western Europe, including westward migration.
A potential history of Amphoracrinus is that this genus originated during the Late Devonian of China, where it thrived in shallow-water, nonbuildup-associated habitats. It then migrated westward, with the first preserved occurrence in western Laurussia (North America) in a siliciclastic deltaic setting (A. viminalis) and the first occurrence in central Laurussia (Western Europe) in a mixed carbonate–siliciclastic setting (A. crassus, A. granulatus, and A. gigas) (Table 1). Younger species in China and North America persisted in mixed carbonate–siliciclastic settings, whereas in Western Europe, Amphoracrinus thrived in facies associated with Waulsortian mudmounds as well as other settings. As noted, the morphological and phylogenetic analyses suggest that migratory pathways were open throughout the Famennian–Viséan interval.
To better resolve the paleogeographic history of Amphoracrinus, a more-detailed understanding of Amphoracrinus sp. from the Famennian of China is required, as well as more occurrences of the genus from both the late Devonian and Tournaisian. Analyses of multiple clades through this interval are also needed to more fully understand this important episode in crinoid evolutionary history.
Taphonomy
Amphoracrinus tenax n. sp. is an illustrative example of the earliest phases of disarticulation of a robust camerate crinoid. Preservation of two sides of this specimen are strikingly different (Fig. 4.2, 4.5). This preservational contrast is well known for many clades with multielement skeletons (e.g., see Brett and Baird, Reference Brett and Baird1986 and Meyer and Milsom, Reference Meyer and Milsom2001 for discussions on this phenomena). This style of preservation has been noted for Paleozoic (Meyer et al., Reference Meyer, Tobin, Pryor, Harrison, Osgood and Roberts1981; Brett and Baird, Reference Brett and Baird1986) and Mesozoic (Springer, Reference Springer1901; Struve, Reference Struve1957; Seilacher et al., Reference Seilacher, Drozdzewski and Haude1968; Hagdorn, Reference Hagdorn1978, Reference Hagdorn1998; Neugebaurer, Reference Neugebaurer1978; Simms, Reference Simms1986; Milsom et al., Reference Milsom, Simms and Gale1994; Hess, Reference Hess, Hess, Ausich, Brett and Simms1999) crinoids.
Brett and Baird (Reference Brett and Baird1986) discussed this differential preservation in the Silurian camerate crinoid Eucalyptocrinites Goldfuss, Reference Goldfuss1831. In this Silurian crinoid, arms were preserved mostly intact on the lower surface, but arms were absent from the upper surface. In both cases, calyx plates were present but displaced due to compaction.
The A. tenax (Figs. 1, 4) holotype USNM PAL 771320 is important for understanding the taphonomy of the crown and the significant height of the column. Further, this allows consideration of relative disarticulation of nearly the entire skeleton of a crinoid inferred to have had only ligamentary articulations. The CD interray is very well preserved, whereas the opposite side (A ray) is in a much more advanced stage of disarticulation. The A ray was the stratigraphically up side when this specimen was found (Fig. 4.1, 4.2). On the A-ray side, the radial plates and fixed brachials remain firmly sutured, but the interradial plates are slightly askew (Fig. 4.5). Every free arm is disarticulated from the calyx. Most of the preserved arms are in approximately the correct position, but the biserial brachials are beginning to separate along some arm segments. In other places, brachials are completely disarticulated but remain as part of the specimen. Pinnules are in the correct position and articulated, in their correct position but slightly askew, or completely disarticulated. By contrast, the CD interray, the stratigraphically down side of the specimen, was buried in the sediment. Calyx plates are sutured; all free arms are in place, with three having minor displacement (Fig. 4.1, 4.2). Proximal arms are mostly intact, but in the middle and distal parts of the arms some disarticulation is present. However, the distal, narrower, incurled arms are intact. A few pinnules are intact, but they are mostly disarticulated. Pinnules may be approximately in place or completely disarticulated. The proximal and middle column are completely articulated, but the distal column is absent (Figs. 1, 4.3, 4.4)
Actualistic studies of disarticulation and preservation have been conducted with articuliform crinoids that have muscles in their arms, and muscles are the most rapidly decaying tissue (Meyer, Reference Meyer1971; Liddell, Reference Liddell1975; Meyer and Oji, Reference Meyer and Oji1992). By contrast, Mississippian camerate crinoids are inferred to have had only ligamentary connective tissues, on the basis of both brachial articulations and disarticulation patterns (e.g., Lane and Burke, Reference Lane and Burke1976; Ausich and Baumiller, Reference Ausich and Baumiller1993).
A possible scenario for the preservation of USNM PAL 771320 is that the crown and 230 mm of the column broke away from the more-distal column, presumably by a storm or gravity flow (Taylor and Brett, Reference Taylor and Brett1996). The crinoid was transported some unknown distance and was only partially buried. The enclosing matrix is uniformly fine grained, so if it was displaced by a storm or gravity flow it was likely transported in the upper, finer-grained portion of the resulting deposit, as discussed by Ausich and Meyer (Reference Ausich and Meyer1990), Taylor and Brett (Reference Taylor and Brett1996), and Ausich (Reference Ausich2021b). The upper, more-exposed surface decayed and disarticulated more rapidly, and the lower buried surface may have decayed more slowly. The specimen was eventually permanently buried before it was completely disarticulated. Current action did not disperse skeletal elements, and it does not appear to have been significantly attacked by scavengers. However, note the lack of plates on the lower arms (left side of Fig. 4.1, 4.2 and the opposite side of Fig. 4.5). It is possible that after burial, this specimen was disturbed by a burrowing organism (Maples and Archer, Reference Maples and Archer1989) (other trace fossils occur in this unit). Thus, although buried and intact, all connective tissues had decayed. Finally, the specimen was compacted and lithified, yielding its present condition (Figs. 1, 4).
The poor articulation on the upper surface was either a reflection of differential disarticulation on the seafloor or a reflection of differential decay of connective tissues and disarticulation manifested by compaction. Overall, the pinnules are the elements displaying the most disarticulation, followed by the arms, calyx, and column. Camerate calyx plates are traditionally considered to have been cemented to one another more than in other clades during life (e.g., Ubaghs, Reference Ubaghs, Moore and Teichert1978a); however, their more-secure articulations may have been due to stereom interlocking (Gorzelak et al., Reference Gorzelak, Stolarski, Mazur and Meibom2012; Ausich, Reference Ausich2021b). The difference in disarticulation propensity of pinnules, arms, and column may be the result of surface area of ligamentary articulations, with the smaller pinnular facets more likely than the larger brachial facets to disarticulate. The largest ligamentary articulations between columnals are all intact. This result is consistent with studies by Allison (Reference Allison1990) and Breton (Reference Breton1997), who concluded that disarticulation rates among asteroid clades were also correlated with plate size and robustness.
During decay of a dead extant crinoid, Meyer and Oji (Reference Meyer and Oji1992) convincingly demonstrated that some sort of a microbial sheath was responsible for the excellent preservation of slabs of Uintacrinus socialis Grinnell, Reference Grinnell1876, which includes numerous specimens with the down sides very well preserved and the up-side plates disarticulated. Crinoid preservation enhancement by microbial sheaths has been hypothesized in other examples of exceptional preservation (e.g., Seilacher et al., Reference Seilacher, Reif and Westphal1985). It is not possible to determine whether a microbial sheath enhanced the preservation of this Muldraugh Member crinoid because, if present, a microbial sheath would not be likely to have been preserved in sediments that lack any evidence of having been anoxic.
Acknowledgments
We thank D. Cooper and B. Cooper of Trilobites of America for preparation of this specimen. G. Monreal is supported in part by a gift from R.M. Prizant to the Legacy Foundation of Kentuckiana. We also thank G.D. Sevastopulo and J.-P. Lin for discussion of stratigraphy and paleogeography in Ireland and China; J.A. Waters for discussion of paleoenvironmental occurrences in China; D.F. Wright for discussion of numerical analyses; and S.R. Cole, T.W. Kammer, and S. Zamora for reviews that improved this manuscript.
Data availability statement
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.jq2bvq889.