Introduction
Cardiliidae bivalves are uncommon and have been rarely mentioned in recent literature. The family name was introduced by Fischer (Reference Fischer1887, p. 1120) and historically placed in the superfamily Mactroidea (Dall, Reference Dall1895; Lamy, Reference Lamy1917; Keen in Moore, Reference Keen1969; Bieler et al., Reference Bieler, Carter and Coan2010; Carter et al., Reference Carter2011, among others). Diagnostic characters are a cordiform shell with a typical mactrid hinge, internal resilium and external ligament, posterior adductor muscle placed into a myophore and the absence of pallial sinus. Currently six extant and fourteen exclusively fossil species have been mentioned in the literature (Lamarck, Reference Lamarck1819; Newton, Reference Newton1891; Deshayes, Reference Deshayes1844; Nicklès, Reference Nicklès1955; Hörnes, Reference Hörnes1859; Fischer, Reference Fischer1861; Oostingh, Reference Oostingh1934; Otuka, Reference Otuka1934; Beets, Reference Beets1944; Van Regteren Altena and Beets, Reference Van Regteren and Beets1945; Tsuda, Reference Tsuda1959, among others). Fossil material representing extinct species has been registered from several localities of Europe and Asia whereas fresh (partially live) specimens (representing extant species) were sampled from the Indo-Pacific and the eastern Atlantic Ocean. Only for one extant species a fossil record exists, which is restricted to the Quaternary.
During an ongoing revision of the superfamily Mactroidea it became necessary to give a synopsis of the current taxonomy and distribution of recent and fossil cardiliids.
Materials and methods
This study is based on an exhaustive literature search of described species belonging to the family Cardiliidae. All original descriptions were checked. Geographical distribution, stratigraphic range, type material and type locality of each extant and fossil species are provided. Valid species herein revised are listed in a stratigraphic chart with temporal and geographic distribution (Table 1).
Table 1 Stratigraphic chart with temporal and geographic distribution of valid species of Cardilia.

*Chart taken from the International stratigraphic chart (ICS) Definition of the Quaternary and revision of the Pleistocene are under discussion. Base of the Pleistocene is at 1.81 Ma (base of Calabrian), but may be extended to 2.59 Ma (base of Gelasian). The historic “Tertiary” comprises the Paleogene and Neogene, and has no official rank.
Repositories and institutional abbreviations
Type material of all nominal species is deposited in the following institutions: Muséum national d’Histoire naturelle (MNHN), Paris; The Natural History Museum (NHMUK), London; Zoologisk Museum (ZMUC), Copenhagen; Natural History Museum if Wien (NHMW); Geology Museum of Bandung, Indonesia (GMBI); Naturalis Biodiversity Center (RGM). Museum of the Institute of Geology and Mineralogy of Faculty of Science, University of Kyoto (JC); the University Museum, The University of Tokyo (UMUT). Where possible this type material was reviewed by the authors.
Systematic paleontology
Superfamily Mactroidea
Family Cardiliidae Fischer, Reference Fischer1887
Diagnosis
Shell small, equivalve, higher than long, extremely inflated, slightly inequilateral, ventrally extended; dorso–ventrally sub–ovate; anteriorly ovate to broadly ovate, dorsally strongly sloping downward anteriorly and posteriorly; ventrally ovate. Posteroventral shell margins slightly digitate in some members. Sculpture of only growth lines or closely spaced, rounded radial ribs over the posterior part of the shell. Inner shell margins smooth. Shell not gaping. Umbos strongly prosogyrate, dorsally projecting, subspiral. Hinge plate strongly arched; very thin anteriorly and posteriorly, thickened only on strong, restricted projection below beaks, containing both ligamental resilifer and cardinal dentition. Hinge with strong, inverted V–shaped cardinal in the left valve, triangular and weaker lamellar cardinal in the right valve, both positioned on a buttress below the resilifer; one lateral tooth present in the right valve. Lamellar part of the ligament not observed. Fibrous resilium submarginal/internal, triangular to tear–drop shaped, slightly posteroventrally inclined, extending onto ventrally projecting chondrophore in each valve. Anterior adductor muscle scar narrow, elongate, positioned close to the anterior shell margin; posterior adductor muscle scar positioned on strongly projecting myophoric lamina, extending ventrally from the postero-dorsal shell margin. Pallial line entire, without sinus. Shells aragonitic and internally porcelaneous, but details of shell microstructure unknown (Taylor et al., Reference Taylor, Kennedy and Hall1973).
Remarks
The family Cardiliidae was traditionally classified into the superfamily Mactroidea. However, the examined species of this family do not really resemble other mactroideans. The characters shared with other mactroidean species are the internal resilium as well as the inverted V-shaped cardinal tooth in the left valve, being this last character, the main diagnostic that defines the superfamily. However, the presence of three different ornamental areas (OA) on shell external surface, the strongly inflated umbo, the shell outline and posterior adductor muscle placed into a myophore are exclusive features of cardiliids. Until new material become available for anatomical and molecular studies we prefer to be conservative and retain Cardiliidae as a separate family within Mactroidea.
Genus Cardilia Deshayes in Lamarck, 1835
Type species
Isocardia semisulcata Lamarck, Reference Lamarck1819, by subsequent designation (Deshayes, Reference Deshayes1844). Usually this type designation was ascribed to Herrmannsen, Reference Herrmannsen1846 (e.g., Keen in Moore, Reference Keen1969). However, Deshayes in Lamarck, 1835 placed both Isocardia semisulcata and Isocardia michelini in his new genus Cardilia without type designation. Later Deshayes (Reference Deshayes1844) assigned Isocardia semisulcata as type species of the genus (Huber, Reference Huber2010).
Occurrence
Europe, Indo–Pacific, Japan, South eastern Atlantic; middle Eocene – Recent.
Remarks
The following genus level names are considered in this work as junior synonym of Cardilia: Hemicyclonosta Deshayes in Blainville, 1827 (p. 660; nomen nudum); Hemiclostera Bronn, Reference Bronn1838 (p. 806; error pro Hemiciclonosta Deshayes in Blainville, 1827); Hemicyclostoma Gray, Reference Gray1840 (p. 136; error pro Hemiciclonosta Deshayes in Blainville, 1827); Cardilla Lycett, Reference Lycett1848 (p. 258; error pro Cardilia Deshayes, 1835); Hemicyclodonta Deshayes, Reference Deshayes1850 (p. 251; error pro Hemiciclonosta Deshayes in Blainville, 1827); Hemicycloster Paetel, Reference Paetel1875 (error pro Hemiclostera Bronn, Reference Bronn1838); Leptina Pictet, Reference Pictet1855 (p. 500; based on manuscript by Bonelli) (non. Meigen, Reference Meigen1830, p. 283, obj.). Besides the genus Cardilia and its synonyms, the Oligocene Cardilona Marwick, Reference Marwick1943 (type C. bensoni Marwick, Reference Marwick1943) had been referred to this family (Keen, Reference Keen1969, p. N608). However, it is currently assigned to Lyonsiellidae as a junior synonym of Pecchiolia Savi and Meneghini in Murchison, Reference Savi and Meneghini1850 (Maxwell, Reference Maxwell1978; Beu and Maxwell, Reference Beu and Maxwell1990).
Cardilia semisulcata (Lamarck, Reference Lamarck1819)

Figure 1 (1–8) Cardilia semisulcata Lamarck, Reference Lamarck1819: (1, 2) holotype MNHN 23202; (3–8) Cardilia semisulcata, Jousseaume collection, MNHN IM-2014-6485. Abbreviations: l: accesory lamellae; 2b: left cardinal tooth; 3b: right cardinal tooth; LAI: anterior lateral teeth; m: myophore; n: nymph. Scale bar (1, 2) 3 mm; (3–8) 1 cm.
Type material
One syntype MNHN 23202, length 24 mm. Les mers de Nouvelle–Hollande, à l'île St. Pierre et St. François, Australia (locality erroneously registered according to Huber, Reference Huber2010).
Remarks
Cardilia semisulcata has been sampled from north Queensland and Western Australia by Lamprell and Healy, Reference Lamprell and Healy1998. Deshayes (Reference Deshayes1844) recorded it from the Malacca Strait and Lamy (Reference Lamy1917) from the Philippines and Japan. Additional records of this species are from off Port Blair, Andaman Islands (Smith, Reference Smith1906); Persian Gulf (Melvill and Standen, Reference Melvill and Standen1907; Al-Khayat, Reference Al-Khayat1997); and offshore Oman (Oliver, Reference Oliver1995) and Northern Territory, New South Wales (both Australia), Fiji, New Caledonia, Papua New Guinea (Atlas of Living Australia). Cotton (Reference Cotton1961, p. 20) stated that this species is not present in South Australia from where it was described, and also the Atlas of Living Australia does not provide any records from that area. Huber (Reference Huber2010) concluded that the type locality is erroneous.
Cardilia atlantica Nicklès, Reference Nicklès1955

Figure 2 (1–3) Cardilia atlantica Nicklès, Reference Nicklès1955, syntype, ZMUC 1512; (4–6) Cardilia bruneiana Beets, Reference Beets1944, holotype RGM 783503; (7, 8) Cardilia deshayesi Hörnes, Reference Hörnes1859, holotype NHM Wien 1855/XLV/286. Scale bar (1, 2) 3 mm; (4–6) 1 cm; (7, 8) 2 mm.
Type material
One syntype, ZMUC 1512, one right valve collected from muddy bottom; collection date: 15/1/1946; height 14 mm, length 8.6 mm, width 5.5 mm. Station 70 of Danish Atlantide expedition to West Africa, Lat: 4.83 N, Long: 2.82 W, 60–65 m depth.
Remarks
Cardilia atlantica Nicklès, Reference Nicklès1955 is a valid living species that comes from the eastern Atlantic Ocean. It was reported by Nicklès (Reference Nicklès1955) as fossil from Quaternary deposits of Port-Gentil, Gabon and as living specimens from Liberia, Ghana (then called Gold Coast) and Nigeria. Similarities with C. inermis Deshayes from the Pacific Ocean and to C. krawangensis Oostingh from the Neogene of Java were mentioned, although Oostingh’s species has a larger shell with a less prosogyrous umbo.
Cardilia bruneiana Beets, Reference Beets1944
Type material
Holotype RGM 783503, articulated specimen; height 20 mm; length 14.7 mm; width 18 mm.; one paratype RGM 783535, height 9.4 mm. Late Miocene deposits (Seria Formation) sample Tutong 13, NW of Tutong, Brunei, NW Borneo. The paratype is from sample Tutong 15B slightly lower in the same formation. The Seria Formation was deposited during the Tortonian as was recently reconfirmed by microfossils from an outcrop of a younger and shallower part of the formation (Bukit Ambug, latest Tortonian; Roslim et al., Reference Roslim, Briguglio, Kocsis, Cori’c and Razak2016).
Remarks
Cardilia bruneiana was registered from deposits of similar age than those where C. krawangensis and C. ludwigi came from. The differences compared to other Indonesian species have been analyzed (Beets, Reference Beets1944). In his publication, two new names were introduced: Cardilia bruneiana and C. palembangensis. The first species has been described from Tutong, Brunei and has a less oval shell than C. krawangensis, with the radial ribs of the posterior area extending further forward ventrally but less so on the umbo. In addition, two undetermined species were mentioned by Beets (Reference Beets1944): Cardilia sp. 1 from Padang, Sumatra and Cardilia sp. 2 from Rembang, Central Java Province.
Cardilia deshayesi Hörnes, Reference Hörnes1859
Type material
Holotype NHM Wien 1855/XLV/286, a single specimen collected from middle Miocene deposits of the Vienna Basin; height 9 mm, length 6 mm, and width 8 mm. Badenian, middle Miocene deposits exposed at Steinebrunn, Vienna Basin, Austria.
Remarks
Cardilia deshayesi is a valid species widely recorded from Badenian (middle Miocene) exposures in the Vienna Basin (Karrer, Reference Karrer1877; Handmann, Reference Handmann1888; Sieber, Reference Sieber1958; Schultz, Reference Schultz2003, among others). It was additionally registered from different localities in east Europe like Smarzowa, Poland (Friedberg, Reference Friedberg1936), Lontov, Slovakia (Tejkal et al., Reference Tejkal, Ondrejíčková and Csepreghy–Meznerics1967), Mikulov, Czech Republic (Studencka et al., Reference Studencka, Gontsharova and Popov1998), and Venetia, Italy (Stefanini Reference Stefanini1916; Venzo, Reference Venzo1934).
Cardilia gemmulata Gould, Reference Gould1861
Type material
Lost, not reported in the original description as deposited in any American institution (Johnson, Reference Johnson1964), height 2.5 mm, length 2 mm, width 2 mm. China seas.
Remarks
Cardilia gemmulata was described from the China Sea, only 2 mm length. It should not be considered a nomen oblitum as concluded by Higo et al. (Reference Higo, Callomon and Goto1999) because it was mentioned after 1899 (art. 23.9.2 of the code) by several authors (Lamy, Reference Lamy1917; Johnson, Reference Johnson1964; Ruhoff, Reference Ruhoff1980; Huber, Reference Huber2010). However, the type material is probably lost as it was not mentioned by Johnson (Reference Johnson1964) as deposited in any institution. Cardilia gemmulata should be considered nomen dubium, as suggested by Huber (Reference Huber2010), until new material can be collected.
Cardilia inermis Deshayes, Reference Deshayes1844

Figure 3 (1−3) Cardilia inermis Deshayes, Reference Deshayes1844, original illustrations; (4, 5) Cardilia martini Deshayes, Reference Deshayes1844, original illustrations. Scale bar 5 mm.

Figure 4 (1–4) Cardilia inermis Deshayes, Reference Deshayes1844, NHMUK 20160445; (5–13) Cardilia martini Deshayes, Reference Deshayes1844, NHMUK 20160446. Scale bar (1, 2) 5 mm; (3, 4) 2 mm; (5–13) 10 mm.
Type Material
Not found. Coast of Sumatra, Indonesia.
Additional material examined
Four valves, two broken, NHMUK 20160445, H. Cuming collection.
Remarks
According to Huber (Reference Huber2010), the species C. inermis is the smaller and more squarish living species. Originally described from Sumatra, its distribution has been expanded to South China, Thailand and Philippine Islands (Fischer, Reference Fischer1861; Lynge, Reference Lynge1909).
Cardilia krawangensis Oostingh, Reference Oostingh1934

Figure 5 (1, 2) Cardilia krawangensis Oostingh, Reference Oostingh1934, original illustrations; (3, 4) Cardilia ludwigi Oostingh, Reference Oostingh1934, original illustrations; (5–14) Cardilia laeviuscula J. de C. Sowerby in Dixon, Reference Dixon1850; (5, 6) Lectotype; (7–14) additional examined material NHMUK 73007b; (15, 16) Cardilia michelini Deshayes in Michelin, 1825, MNHN A09474, syntype, image photographed by Jacques Mouchart (Project: E–Recolnat). Scale bar (1–14) 5 mm; (15, 16) 5 mm.
Type material
Not found, probably deposited at GMBI; height 11.5 mm, width 7.5 mm, thickness 10.5 mm,. Besides the holotype, there are two specimens from the same locality. Topographical/Geological Map Sheet 30 (Purwakarta, Java Province, Indonesia), Locality 1998, Cikao, Cidadap Formation (previously known as Tjidadap Beds, late Miocene to early Pliocene, sensu Dr. O. Ludwig).
Remarks
Although C. krawangensis superficially resembles C. palembangensis, it differs clearly in shape, having a more rounded posterior side and being considerably smaller. The escutcheon is broader and smooth. The number of ribs is higher and they do not reach the middle of the shell along the ventral side as in C. palembangensis.
Cardilia laeviuscula Sowerby in Dixon, Reference Dixon1850
Type material
Lectotype herein designated: NHMUK PI OR 73007b, the specimen figured in Dixon, Reference Dixon1850 (pl. 2, fig. 6a; three views); paralectotypes: three specimens NHMUK PI OR73007a from Bracklesham Bay, middle Eocene. Locality: Selsey, Bracklesham Bay, West Sussex, England. Lithostratigraphy: Unit S10 (Curry et al., 1978), Selsey Formation, Bracklesham Group; confined to this horizon (J. Todd, personal communication, 2017). Age: late Lutetian, zone NP16, middle Eocene.
Remarks
In this work, two species from the fossil record of England are considered valid. The first one is Cardilia laeviuscula described by Sowerby in Dixon, Reference Dixon1850 (p. 165, pl. 2, fig. 6a) from middle Eocene deposits exposed at Bracklesham Bay, West Sussex, UK. The second one is Cardilia edwardsi n. sp.
Cardilia ludwigi Oostingh, Reference Oostingh1934
Type material
Not found, probably deposited at GMBI; height 9.5 mm, width 7.8 mm thickness 8.7 mm. Two additional specimens registered from the same locality. The hard molds do not allow observation of the hinge. Purwakarta, Java province, Indonesia, Locality 1998, Cikao, Cidadap Formation (previously known as Tjidadap Beds), late Miocene to early Pliocene, sensu Dr. O. Ludwig.
Remarks
Cardilia ludwigi has a circular outline with finer radial ribs and narrower interspaces. Oostingh’s species, C. krawangensis, and C. ludwigi, came from one outcrop and thus have the same age and lived as sympatric species. Their age is very similar to that of C. bruneiana. The descriptions of these two species are based on Oostingh’s descriptions and drawings because the type material could not be accessed.
Cardilia martini Deshayes, Reference Deshayes1844
Type material
Not found. Malacca Strait, SE Asia.
Additional material examined
Three valves, one broken, NHMUK 20160446. Measures of largest specimen: height 16 mm, length 12 mm.
Remarks
Cardilia martini, described from the Malacca Strait has been recorded from the coast of China and the Philippines (Adams and Adams, 1853–Reference Adams and Adams1858; Sowerby, Reference Sowerby1873; Paetel, Reference Paetel1883). It is the narrowest living species with stronger ribs (Huber, Reference Huber2010). However, no modern records of this species were found in the literature.
Cardilia michelini (Deshayes in Michelin, Reference Deshayes1825).
Type material
MNHN A09474, one well-preserved syntype. La Chapelle-en-Serval, Oise, France, Sables de Beauchamp, Bartonian (Auversien), middle Eocene. Syntype measures: height 16 mm, length 11 mm.
Remarks
Cardilia michelini was introduced by Deshayes in Michelin (Reference Deshayes1825) and illustrated on a lithographic plate with its respective explanation. Sensu Keen (Reference Keen1969), the evidence for valid publication by Michelin is inconclusive, however his contemporaries accepted it. Later, C. michelini was recorded as fossil in France by other authors (Deshayes, Reference Deshayes1827; Deshayes and Milne Edwards, Reference Deshayes and Milne Edwards1835; Fischer, Reference Fischer1861; Cossmann and Pissarro, Reference Cossmann and Pissarro1906; Le Renard and Pacaud, Reference Le Renard and Pacaud1995). It was additionally reported from the Eocene of Gerona, Spain (Marcet I Riba, Reference Marcet1955).
Cardilia michelottii Deshayes, Reference Deshayes1844
Type material
Not found. Type measures reported in the original description: height 23 mm, length 18 mm, width 20 mm. Collected by M. Michelotti from Pliocene deposits of Asti, Piedmont, Italy.
Remarks
Cardilia michelottii is a valid species subsequently registered by several authors from the Piacenzian, late Pliocene of Italy (Bronn, Reference Bronn1848; d´Orbigny, Reference Orbigny1852; Manzoni, Reference Manzoni1868; Pantanelli, Reference Pantanelli1892; Sacco, Reference Sacco1901).
Cardilia palembangensis Beets, Reference Beets1944

Figure 6 (1–12) Cardilia palembangensis Beets, Reference Beets1944 type series from Talang Alab, Palembang; (1−4) paratype 1 RGM 822549; (5−8) holotype RGM 822551; (9−12) paratype 2 RGM 1007835. (13–15) Cardilia sundaica Van Regteren Altena and Beets, 1945, holotype RGM 820549. (16) Cardilia toyamaensis Tsuda, Reference Tsuda1959, holotype, left valve JC1400026. OA: Ornamental area. Scale bar (1–16) 10 mm.
Type material
Originally deposited at Geological Museum, University of Utrecht, now RGM. Holotype RGM 822551, articulated specimen height 16.4 mm, length 12.5 mm, width 14.8 mm; RGM.822549 paratype 1; RGM.1007835 Paratype 2 (single valve); RGM.1007836 a paratype, single valve (part of larger sample of paratypes with number RGM.1007836, containing three articulated specimens and this single valve). Test pit Talang Abab, province South Sumatra, Indonesia; Air Benakat Formation, Lower Palembang Member; middle to late Miocene.
Remarks
Table 2 compares the five species from SE Asia. Of all the SE Asian fossil species, the largest number of specimens is available for C. palembangensis. Besides the four articulated specimens and two valves in the sample from the type locality, 15 articulated specimens were collected from the Pendopo oilfield, 10 miles E of Talang Akar, early to middle Miocene, border between lower and upper part of the Telisa Formation, sensu Huysse, 15 articulated specimens, RGM820.364 and one articulated specimen from the Kampong Tengah C/D outcrops, S. of Miri, Sarawak, Malaysia, Sibuti Formation, Langhian, middle Miocene (sample F41.23 in J.G.M. Raven’s collection). Because there are adult and juvenile specimens, the variation in this species is well understood (Table 3 gives measurements for a selection of these). Of the other species from this area, only specimens of Cardilia sundaica are available. Additional material will help both in the differentiation among the species and understanding their stratigraphic ranges.
Table 2 Morphological comparison of the five extinct species from SE Asia.
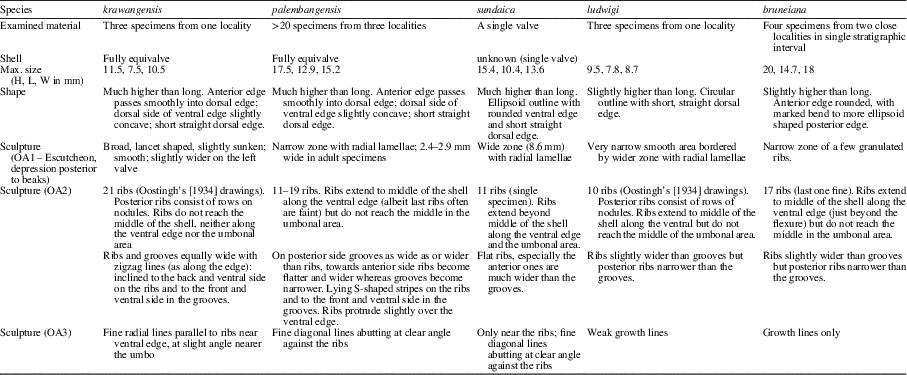
Table 3 Measurements of C. palembangensis showing its intraspecific variation and comparison with C. sundaica. Size measurements in mm.

Cardilia reeveana Hidalgo y Rodriguez, Reference Hidalgo y Rodriguez1903
Type material
Does not exist. The taxon is based on Sowerby´s illustration (in Reeve and Sowerby, 1843–1878, pl. 1, fig. 2) of Cardilia inermis (non Deshayes). Philippines.
Remarks
Cardilia reeveana Hidalgo y Rodriguez, Reference Hidalgo y Rodriguez1903, based on Sowerby´s illustration of C. inermis, seems to be unnecessary. Huber (Reference Huber2010) noted that Sowerby´s illustration was accepted by Lynge (Reference Lynge1909) as belonging to the earlier described C. inermis, which expanded the distribution of that species previously given by Fischer (Reference Fischer1861) from southern China and east Thailand, to also include the Philippines.
Cardilia sundaica Van Regteren Altena and Beets, Reference Van Regteren and Beets1945
Type material
Holotype RGM 820549, single valve, height 15.2 mm, length 11 mm. Originally the type was stored at the Geological Institute, University of Amsterdam; it is now at RGM. Left bank Ci Gugur River, north of Koleberes plantation, SW of Bandung province, West Java, Indonesia; late Miocene.
Remarks
Cardilia sundaica Van Regteren Altena and Beets, Reference Van Regteren and Beets1945 was recognized by its authors as an intermediate form between C. ludwigi and C. krawangensis. It has a narrower anterior surface (AO3) without shell sculpture, delimited posteriorly by radial ribs that are transversely wrinkled. The furrows and ridges are all approximately equal in width, but the ribs are narrower than the furrows. The lunule is significantly thinner than in C. krawangensis. The fine radial shell sculpture runs almost parallel to the ribs, but is much narrower.
Cardilia toyamaensis Tsuda, Reference Tsuda1959
Type material
Holotype, left valve JC1400026. Type measures: height 11.7 mm, length 9 mm, thickness 5.5 mm; three paratypes JC 1400027, JC140028, one right valve and two contact valves. Kurosedani Formation, late early Miocene. Toyama Prefecture on the new River County Daze, Japan.
Remarks
The Japanese Cardilia toyamaensis appears to be common because it has been recorded from different Japanese Miocene outcrops (Okamoto and Terachi, Reference Okamoto and Terachi1974; Takayasu, Reference Takayasu1981; Taguchi, Reference Taguchi2002; Nakagawa, Reference Nakagawa2009, among others). This species differs from the recent C. semisulcata in its smaller shell without radial sculpture on the posterior area and in its smaller number of ribs.
Cardilia edwardsi new species

Figure 7 (1–5) Cardilia edwardsi n. sp. from the Becton Formation (late Bartonian) of Barton, Hampshire, UK; (1) holotype, NHMUK PI TB 14589, (2) paratype, one broken shell, NHMUK PI TB 14589, both from the Curry collection; (3, 5) NHMUK PI OR 73008; (4) NHMUK PI OR 73008, additional material from the F.E. Edwards collection. Scale bar (1–5) 5 mm.
Type material
Holotype, NHMUK PI TB 14589 (1), length 5.4 mm, height 5.6 mm; paratype, one broken shell, length 7.3 mm, height 7.6 mm, NHMUK PI TB (2), both Dennis Curry collection.
Diagnosis
Shell small, radial ribs crossed by fine concentric striation, disposed along the postero-dorsal axis from the umbo to the ventral edge, external surface areas poorly defined; on the anterior side of this line the external surface is reticulated over the umbonal area, and with fine and irregular growth lines along the ventral edge; posterior myophore well developed, trigonal with a circular posterior adductor muscle scar.
Occurrence
Type locality: Barton–on–Sea, Hampshire, England. Lithostratigraphy: ‘Chama Bed’ (Bed H of Burton, 1933), Becton Formation, Barton Group. Age: late Bartonian, middle Eocene.
Description
Shell small, fragile, subcircular, height up to 8 mm; external surface with three areas; dorso–posterior, with irregular and small concentric striation from the posterior edge to the first ribs of the adjacent area; external area with radial and narrow ribs disposed along the postero-dorsal axis from the umbo to the ventral edge; all ribs crossed by additional shell sculpture of concentric and irregular growth lines; straight line that separates the external surface areas poorly defined; area of the external surface covering a major portion of the shell, composed by fine and irregular growth lines less evident near the ventral edge and reticulated shell sculpture over the umbonal area,. This area extends from the straight line towards the anterior side of the shell; internally mactroid hinge plate with an oblique chondrophore ventrally projected and a posterior trigonal myophore well developed, with a circular posterior adductor muscle scar well visible; pallial sinus absent.
Etymology
Honoring Frederick E. Edwards, the discoverer of this species.
Remarks
This species was mentioned previously by a few authors as Cardilia radiata from exposures of the Barton and the Bracklesham Beds (Tennant, Reference Tennant1847, p. 29; Beet–Jukes, Reference Beete Jukes1857, p. 530; Newton, Reference Newton1891, p. 85). Although this species was attributed to either Edwards or Sowerby, it is not listed by Petit (Reference Petit2009) as a taxon named by Sowerby and not found published in the “Quarterly Journal of the Geological Society of London” by Edwards (Reference Edwards1854). Cardilia radiata appears in a manuscript name of F.E. Edwards that was never validated. In this work, it is formally described as Cardilia edwardsi new species from the Becton Formation, Barton Group.
Discussion
Literature search allows an understanding of the geographical and temporal radiation of the family Cardiliidae. Extant species were historically sampled from the western Pacific Ocean in the Philippines, Indonesia, China, and Malaysia and the Indian Ocean/Persian Gulf in Oman and Qatar. From this huge area, only three valid species are recognized: C. semisulcata, C. inermis, and C. martini. Cardilia atlantica is the fourth living species registered in the Atlantic Ocean. On the other hand, from 14 names found in the literature for species only known as fossils, 11 are recognized as valid. Five valid species were registered from the fossil record of Europe, one of them formally described herein. They were registered from different localities in England, France, Austria, Italy, and Russia, among others. The stratigraphic range of these records goes from middle Eocene to late Pliocene. They are: Cardilia deshayesi from middle Miocene exposures at Steinebrunn, Vienna Basin, Austria; Cardilia michelottii from Pliocene deposits at Asti, Italy; Cardilia michelini described from Bartonian (middle Eocene) deposits exposed at La Chapelle-en-Serval, Oise, France; Cardilia laeviuscula from Lutetian (middle Eocene) strata, Bracklesham Bay, West Sussex, England; and Cardilia edwardsi n. sp. from the Becton Formation, late Bartonian, middle Eocene of Hampshire, England.
Finally, the family Cardiliidae was recorded in the fossil record of Asia from where seven species have been described: four from Indonesia, one from Brunei and two from Japan. These are C. sundaica Van Regteren Altena and Beets, Reference Van Regteren and Beets1945; C. bruneiana and C. palembangensis, both described by Beets in 1944; C. krawangensis and C. ludwigi, both described by Oostingh in 1934; and Cardilia yudaensis Otuka, Reference Otuka1934 and Cardilia toyamaensis Tsuda, Reference Tsuda1959 from Japan. The species Cardilia yudaensis, described by Okuta (1934, p. 620, pl. 48, figs. 46–48), was reported from lower Kadonosawa Series, Yuda, Iwate Prefecture, Japan. The author placed this species with doubts within the genus Cardilia, although he considered it as the most adequate. However, we conclude that after revision of the original description and illustration, the species Cardilia yudaensis must be excluded from the genus Cardilia due to the inequivalve form of the type material.
Two nominal subspecies (Cardilia michelini asiana and Cardilia michelini georgiana) are recorded in the Global Names Index portal (http://www.gni.globalnames.org/name_strings), as described by Korobkov (Reference Korobkov1971). However, these names are not found within the publication (S. Popov, personal communication, 2016). In addition, type material of either taxon has not been deposited into the collection of Saint Petersburg Central Geological Museum, where Korobkov´s types are (S. Popov, personal communication, 2016). Therefore these names are considered invalid.
All species belonging to the genus Cardilia have three different ornamental areas (OA) on the external surface of the shell. The first one (OA1) is the escutcheon: dorso–posterior, concentric, uniformly separated, lamellar, nodular towards the top, and intersecting the first ribs of the second ornamental area. It extends from the posterior side of the umbo to the mid-posterior end of the shell. The second area (OA2) has narrow ribs disposed along the postero-dorsal axis from the umbo to the ventral edge and is delimited by a straight line that separates this area from the contiguous one. The number of ribs of this area is variable. The third and final area (OA3) covers almost the entire external surface and is composed fine and irregular growth lines that become invisible along the ventral edge. This area extends from the straight line towards the anterior side of the shell. The morphologies of these areas have taxonomic value for the identification of the different species of the genus. In addition, the outline of the shell plays an important role in the identification of Cardilia species. Hinge morphology of all examined species is very conservative with the strong inverted V–shaped cardinal tooth obliquely anteriorly directed in the left valve, which is complementary to the prominent, triangular, and bifid cardinal tooth (3a) of the right valve; additionally, with a second cardinal tooth (3b) in the right valve and a small and rudimentary anterior lateral tooth (LAi).
Many works had been revised morphologically and genetically the phylogeny of the related families Mactridae and Mesodesmatidae (Giribet and Wheeller, Reference Giribet and Wheeler2002: Taylor et al., Reference Taylor, Williams, Glover and Dyak2007; Combosch et al., Reference Combosch, Collins, Glover, Graf, Harper, Healy, Kawauchi, Lemer, Mcintyre, Strong, Taylor, Zardus, Mikkelsen, Giribet and Bieler2017, among others). The superfamily Mactroidea was traditionally considered to be a well-supported monophyletic group with a sister group, Cardioidea. Taylor et al. (Reference Taylor, Williams, Glover and Dyak2007) agreed with the monophyletic concept of Mactroidea, but suggested a potential sister group with Ungulinidae, and the group Veneridae/Corbiculidae/Arcticidae, and Chamidae, but with no connection with Cardioidea. Within this context, the phylogenetic position of Cardiliidae within Mactroidea is uncertain.
The first records of mactrids date from the Early Cretaceous (Aptian). In a detailed study of hinge development, Saul (Reference Saul1973) suggested that mactrids are derived from the Arcticidae. Cretaceous Mactridae are characterized by a moderate development of resilifer with two cardinals in each valve and long laterals. This kind of hinge was also observed in the Arcticidae, however in Cenozoic Mactridae this similarity is masked due to the enlargement of the resilifer. The systematic position of Cardiliidae within Mactroidea was historically based on similarities of the hinge structure and resilifer. As noted by Keen (in Moore, 1969) and subsequent authors, these similarities include a similar pattern of cardinal and lateral teeth with an external ligament small or wanting, and an internal ligament seated in a resilifer.
There are no new data on anatomy, reproduction, and ecology of Cardiliidae. Thus, the conventional position within Mactroidea is unconfirmed, but retained. Besides type material of described species, scarce additional specimens are available in malacological collections. The position of Cardilia between Mactridae and Myidae, mentioned by Marwick (Reference Marwick1943), was never tested. Currently, the superfamily Mactroidea includes four families (Signorelli and Carter, Reference Signorelli and Carter2016). They are Mactridae, which originated in the Late Cretaceous, Anatinellidae, Mesodesmatidae, and Cardiliidae, all recorded from the Eocene. All families are characterized as shallow or deep burrowers, occurring in the intertidal zone or on the shelf.
This work is part of an ongoing revision of superfamily Mactroidea, including all recent and fossil species. This study includes morphological data both from literature and from new observations of type material and non-type specimens, and states the basis for future studies on recent and fossil species of Cardiliidae.
Conclusions
The Cardiliidae comprise a single genus, Cardilia, which is known from middle Eocene to Recent. Most species are rare and material available is very limited. Eleven extinct species (including one that is described in this paper) and four extant species (for which fossil material is remarkably scarce) are recognized. The placement of the family Cardiliidae in the superfamily Mactroidea requires confirmation.
Acknowledgments
Special thanks to V. Herós and P. Maestrati (MNHN); A. Salvador, K. Way, and J. Todd (NHMUK); T. Schiøtte (ZMUC); B. Hoeksema, F. Wesselingh, and R. Pouwer (Naturalis); and M. Harzhauser and O. Mandic (NHMW) for their assistance in the revision of type material and additional examined specimens. Many thanks to G. Rosenberg and S. Popov for their comments. JHS acknowledges CONICET of Argentina. This work was partially supported by the PICT 640 of the Agencia Nacional de Investigaciones Científicas y Técnicas. This is publication N° 89 of the Laboratorio de Reproducción y Biología Integrativa de Invertebrados Marinos (LARBIM).












