Introduction
Dental mesowear is a useful tool to infer the diet of extinct herbivores. It was originally developed for herbivorous mammals with lophodont teeth and labiolingual mandibular excursion, and it was based on the analysis of the relief (high or low) and shape (sharp, rounded, or blunt) of the ectoloph’s second upper molar cusps (Fortelius and Solounias, Reference Fortelius and Solounias2000). Later, the original method was extended to include analysis of the upper third molar in ruminants (Franz-Odendaal and Kaiser, Reference Franz-Odendaal and Kaiser2003). Also, three univariate scales of dental wear have been developed to facilitate the comparison between mesowear patterns; one ranging from 0 to 3 is used for upper dentition (Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b), the second from 0 to 4 is employed for upper postcanine dentition (Kaiser, Reference Kaiser2011; Kaiser et al., Reference Kaiser, Müller, Fortelius, Schulz, Codron and Clauss2013), and the other from 1 to 5 is used for both upper and lower teeth (Fraser et al., Reference Fraser, Zybutz, Lightner and Theodor2014). Recently, mesowear III was developed to analyze the lingual enamel band of the paracone and metacone; this method has a scoring system that considers four wear stages (1 to 4) of the mesial and distal enamel bands, and the shape of its junction (also 1 to 4), where each stage is associated with the attrition signal, linked to a particular dietary guild (Solounias et al., Reference Solounias, Tariq, Hou, Danowitz and Harrison2014). The original mesowear score, its modifications, and the mesowear III method can be used together to better predict ruminant diet than any single variable (Danowitz et al., Reference Danowitz, Hou, Mihlbachler, Hastings and Solounias2016).
Mesowear is considered one of the main dietary approaches to infer diet (Fortelius and Solounias, Reference Fortelius and Solounias2000; Hoffman, Reference Hoffman2006; Semprebon and Rivals, Reference Semprebon and Rivals2007; Rivals et al., 2009a, Reference Rivals, Schulz and Kaiserb; Blondel et al., Reference Blondel, Merceron, Andossa, Taisso, Vignaud and Brunet2010), along with other approaches, such as stable carbon isotope analysis and dental microwear. In ruminants, mesowear provides additional information, such as geographical (Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b) and temporal variation of diet (Rivals and Semprebon, Reference Rivals and Semprebon2006; Fraser and Theodor, Reference Fraser and Theodor2013).
One of the most conspicuous ruminants in the North American Pleistocene was the bison (Bison spp.); it was the largest, most numerous, and the most widely distributed ruminant (McDonald, Reference McDonald1981; Shapiro et al., Reference Shapiro, Drummond, Rambaut, Wilson, Matheus, Sher, Pybus, Gilbert, Barnes, Binladen, Willerslev and Hansen2004). Bison ranged from Baillie Island in the Yukon, Canada (Harrington, Reference Harrington1990) to Matagalpa, Nicaragua (Howell and MacDonald, Reference Howell and MacDonald1969). Across this wide geographical range, bison mesowear studies are scarce, and are focused on topics such as diet quality in Bison bison (Linnaeus, Reference Linnaeus1758) (Berini, Reference Berini2010), ontogenetic diet variation in B. priscus (Bojanus, Reference Bojanus1827) (Rivals et al., Reference Rivals, Mihlbachler and Solounias2007a), and geographical diet variation in some species of the genus (Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b). Such studies were performed with fossil samples from middle and high latitudes of North America, whereas the diets of the southern bison species are unknown.
Five species of Bison have been reported from the Quaternary of Mexico: Bison alaskensis Rhoads, Reference Rhoads1897, B. antiquus Leidy, Reference Leidy1852, B. bison, B. latifrons (Harlan, Reference Harlan1825), and B. priscus (see Arroyo-Cabrales et al., Reference Arroyo-Cabrales, Polaco and Johnson2002; Ferrusquía-Villafranca et al., Reference Ferrusquía-Villafranca, Arroyo-Cabrales, Martínez-Hernández, Gama-Castro, Ruiz-González, Polaco and Johnson2010). Of these species, B. bison appears between 4 and 5 ka, during the early Holocene in southern Canada (Wilson et al., Reference Wilson, Hills and Shapiro2008). Among Pleistocene species, B. antiquus has the widest geographic range in Mexico, with fossil material from Baja California (Skinner and Kaisen, Reference Skinner and Kaisen1947; Ferrusquía-Villafranca and Torres-Roldán, Reference Ferrusquía-Villafranca and Torres-Roldán1980; McDonald, Reference McDonald1981), Baja California Sur (McDonald, Reference McDonald1981), Estado de México (Hibbard, Reference Hibbard1955), Jalisco (Downs, Reference Downs1956; McDonald, Reference McDonald1981; Lucas, Reference Lucas2008), Puebla (McDonald, Reference McDonald1981), San Luis Potosí (Skinner and Kaisen, Reference Skinner and Kaisen1947; McDonald, Reference McDonald1981), Michoacán (Díaz-Sibaja, Reference Díaz-Sibaja2013), and Oaxaca (Jiménez-Hidalgo et al., Reference Jiménez-Hidalgo, Cabrera-Pérez, MacFadden and Guerrero-Arenas2013).
Because Bison antiquus is the most abundant bison in North America and Mexico and given the scarce knowledge of its dietary patterns in its southernmost range, the main objectives of this paper are: (1) to infer the diet of three Mexican samples of B. antiquus: two from west central Mexico and one from southern Mexico; and (2) to compare it with the diet of B. antiquus from temperate North America and with the modern bison.
Materials and methods
Locality and stratigraphic information
Specimens of Bison antiquus for this study come from three local faunas in west-central and southern Mexico, named La Piedad-Santa Ana, La Cinta-Portalitos, and Viko Vijin (Fig. 1).

Figure 1 Geographic location of the study areas.
La Piedad-Santa Ana is located between the municipalities of La Piedad de Cabadas (Michoacán state) and Pénjamo (Guanajuato state). In the east, its geomorphology is dominated by the alluvial plain of the Lerma River and volcanic hills to the west. Also, there are small extinct monogenetic volcanoes in the north and south of the study area. Stratigraphy (Fig. 2, right) is composed of an alternation of fluvial and lacustrine sequences with some volcanic tuffs at the base, and archaeological elements at the top. Fossils are embedded in a yellowish conglomerate with a sandy matrix (Díaz-Sibaja et al., Reference Díaz-Sibaja, García-Zepeda, López-García, Tejeda-Alvarado, Marín-Leyva and Gutiérrez-Bedolla2012; Díaz-Sibaja, Reference Díaz-Sibaja2013; Marín-Leyva et al., Reference Marín-Leyva, DeMiguel, García-Zepeda, Ponce-Saavedra, Arroyo-Cabrales, Schaaf and Alberdi2016b).
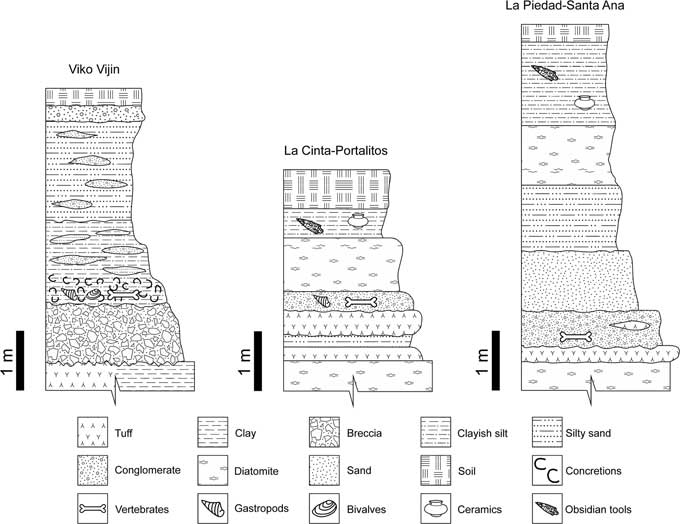
Figure 2 General stratigraphy of the fossil localities in this study.
La Cinta-Portalitos is located between the municipalities of Cuitzeo (Michoacán state) and Uriangato (Guanajuato state). Its geomorphology is dominated by extinct shield volcanoes to the west and northwest, the latter with faults of NE-SW direction. To the north, ash-cinder and maars volcanoes occur, and the Recent bottom of Cuitzeo Lake is to the south. Stratigraphy (Fig. 2, center) is mainly composed of lacustrine sequences, dominated by diatomite beds; between these facies, there are some fluvial beds with sand and some sandy tuffs. At the top, there are archaeological remains. The fossils occur disarticulated in an extensive bed of brown conglomerates with silt and sandy matrix (García-Zepeda, Reference García-Zepeda2006; Marín-Leyva et al., Reference Marín-Leyva, DeMiguel, García-Zepeda, Ponce-Saavedra, Arroyo-Cabrales, Schaaf and Alberdi2016b).
The Viko Vijin local fauna comes from six localities from the municipalities of Concepción Buenavista, San Antonio Acutla, Teotongo, and Villa Tejupam de la Unión, and is located in the northwestern region of Oaxaca state. Area geomorphology is dominated by hills and gullies from the Mixteca Alta Oaxaqueña. Stratigraphy (Fig. 2, left) is composed mainly of fluvial sequences with abundant silty to sandy strata; at the base of the sequence, there are volcanic tuffs or breccias. Fossils occur in clayey and sandy strata with some silt and abundant caliches or nodular calcretes (Jiménez-Hidalgo et al., 2011, Reference Jiménez-Hidalgo, Cabrera-Pérez, MacFadden and Guerrero-Arenas2013).
Taxonomic identification
Selected molars were identified as Bison antiquus by morphological and morphometric comparison with teeth from the literature (Allen, Reference Allen1876; Chandler, Reference Chandler1916; Hillson, Reference Hillson2005), by direct comparison with osteological collections (such as the one at the Laboratorio de Arqueozoología ‘M. en C. Ticul Álvarez Solórzano,’ Instituto Nacional de Antropología e Historia, Mexico City), and by their association with several isolated horn cores and complete skulls of this species in the study localities (McDonald, Reference McDonald1981; Jiménez-Hidalgo et al., Reference Jiménez-Hidalgo, Cabrera-Pérez, MacFadden and Guerrero-Arenas2013).
Mesowear
We selected second (Fortelius and Solounias, Reference Fortelius and Solounias2000) and third upper molars (Franz-Odendaal and Kaiser, Reference Franz-Odendaal and Kaiser2003) of adult individuals in an intermediate wear stage, between stages S-1b to S-3 for M2, and S2 to S4a for M3 (Skinner and Kaisen, Reference Skinner and Kaisen1947). Our sample size consisted of nine specimens for La Cinta-Portalitos, 11 specimens for La Piedad-Santa Ana, and 16 for Viko Vijin (see Supplemental dataset 1). Although it was originally suggested that the minimum number of observations to obtain a robust mesowear signal should be 10 (Fortelius and Solounias, Reference Fortelius and Solounias2000; Kaiser et al., Reference Kaiser, Solounias, Fortelius, Bernor and Schrenk2000), later studies suggested that smaller sample sizes are sufficient to offer insights into paleodiet and paleoecology (Bernor et al., Reference Bernor, Semprebon and Damuth2014). Since then, many studies have offered paleodietary and paleoecological inferences with small sample sizes, ranging from nine to three, and even one (Danowitz et al., Reference Danowitz, Hou, Mihlbachler, Hastings and Solounias2016).
For the scoring of mesowear variables (Fig. 3), the labial portion of the ectoloph cusps of the paracone and metacone were observed and categorized by their relief (high or low) and their shape (sharp, rounded, or blunt) (Fortelius and Solounias, Reference Fortelius and Solounias2000). To control interobserver error when scoring relief, we employed the methodological proposal of Merceron et al. (Reference Merceron, Schulz, Kordos and Kaiser2007) in which the relief state (high or low) was obtained by dividing the distance that connects the highest point of the evaluated cusp and the lowest point of the intercuspidal area (Fig. 3.1, x) by the cusp height (Fig. 3.1, y); when the value was greater than two, we considered it as a low cusp. We employed the software ImageJ 1.50i (Ferreira and Rasband, Reference Ferreira and Rasband2012) for these measurements. Also, we scored cusp shape according to the degree of facet development: (1) when no facets were distinguishable, we considered the cusp as blunt; (2) when there was no distinguishable junction between facets, we considered the cusp as rounded; and (3) when this junction was distinguishable, we considered the cusp as sharp (Fortelius and Solounias, Reference Fortelius and Solounias2000; Kaiser et al., Reference Kaiser, Solounias, Fortelius, Bernor and Schrenk2000; Merceron et al., Reference Merceron, Schulz, Kordos and Kaiser2007).

Figure 3 Mesowear variables: (1) categorization of traditional and extended mesowear variables: shape and relief. Relief is considered high when x/y ≤ 2, or low when x/y>2; (2) mesowear III variables: j-junction (where mesial and distal enamel bands meet) and mesial/distal enamel band score system.
We also evaluated the lingual facets (mesial and distal) of the paracone and its junction (Fig. 3.2), categorizing the facets within the following stages: (1) flat surface with no gouges, (2) nearly flat surface with some gouges, (3) rounded surface with abundant gouges, or (4) smooth rounded surface with no gouges and poorly defined edges. The j-junction was categorized in one of the following stages: (1) well-defined, sharp edge; (2) poorly defined edge with some gouges; (3) rounded edge, poorly defined, smooth, but still-differentiated edge; or (4) undifferentiated mesial and distal cusps, and j-junction lost (Solounias et al., Reference Solounias, Tariq, Hou, Danowitz and Harrison2014; Danowitz et al., Reference Danowitz, Hou, Mihlbachler, Hastings and Solounias2016).
We observed the analyzed molars with a stereoscopic microscope to discard those with taphonomical alterations such as mechanical fractures (Fortelius and Solounias, Reference Fortelius and Solounias2000) or abrasion caused by sand (Blondel et al., Reference Blondel, Merceron, Andossa, Taisso, Vignaud and Brunet2010). To minimize subjectivity when recording mesowear variables, we compared our scorings with the original diagrams and photographs of Fortelius and Solounias (Reference Fortelius and Solounias2000) and Danowitz et al. (Reference Danowitz, Hou, Mihlbachler, Hastings and Solounias2016), assuring that we obtained similar results to those of experienced observers, because mesowear is less prone to observer variation than other methods such as dental microwear (Loffredo and DeSantis, Reference Loffredo and DeSantis2014).
To identify the mesowear pattern associated with a particular dietary category (browser, mixed feeder, strict grazer, and nonstrict grazer) of the Bison antiquus samples of this study, we performed a cluster analysis with 27 model species with known dietary preferences taken from the original Fortelius and Solounias (Reference Fortelius and Solounias2000) database with the variables Per-High, Per-Sharp, and Per-Blunt (percentages of high, sharp, and blunt cusps in the total sample), using the complete linkage (without standardization of the data) clustering method, and Euclidean distance for scaling (Fortelius and Solounias, Reference Fortelius and Solounias2000; Franz-Odendaal and Kaiser, Reference Franz-Odendaal and Kaiser2003; Clauss et al., Reference Clauss, Franz-Odendaal, Brasch, Castell and Kaiser2007).
In addition to the cluster analysis, we transformed the original Fortelius and Solounias (Reference Fortelius and Solounias2000) database to the univariate 0−3 scale of mesowear to compare the signatures of our three samples of Bison antiquus with known dietary categories (browser, grazer, and mixed feeder) of model and extinct Bison species as follows: high and sharp cusps were assigned a 0 score, high and rounded a 1, low and sharp or low and rounded a 2, and blunt cusps were assigned a 3 score (Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b). Additionally, we performed Pearson’s chi-squared tests (α=0.05) to compare the mesowear patterns of the 0−3 univariate scale (Fortelius and Solounias, Reference Fortelius and Solounias2000; Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b) and the mesowear III (Solounias et al., Reference Solounias, Tariq, Hou, Danowitz and Harrison2014) with our samples, to determine the similarities in the wear patterns with the model dietary groups (i.e., browser, grazer, or mixed feeder).
Although we recognize that relief and cusp shape can be affected by the creep of phylogenetic signal (Fortelius and Solounias, Reference Fortelius and Solounias2000), independent data suggest that when a phylogenetic control is introduced, the correct classification of a samples improves by only one percent (Fraser and Theodor, Reference Fraser and Theodor2011), and thus, we did not consider use of any phylogenetic control.
Finally, to predict the dietary guild with more accuracy, we combined the data of extended mesowear (0−3 univariate scale) and mesowear III (scores of the mesial, distal, and j-junction) to perform a discriminant analysis (see Supplemental data set 2 for raw data). This combination of analyses can better predict the diet of a fossil sample than just a single variable because they encompass different parts of the chewing cycle (Janis, Reference Janis1990; Danowitz et al., Reference Danowitz, Hou, Mihlbachler, Hastings and Solounias2016). Mesowear univariate data was transformed from those of Fortelius and Solounias (Reference Fortelius and Solounias2000), mesowear III data were taken from Danowitz et al. (Reference Danowitz, Hou, Mihlbachler, Hastings and Solounias2016), and for the Mexican data, an average per sample was employed. Originally, the combination of extended mesowear and mesowear III (Danowitz et al., Reference Danowitz, Hou, Mihlbachler, Hastings and Solounias2016) employed the 0−6 univariate mesowear scale developed by Mihlbachler et al. (Reference Mihlbachler, Rivals, Solounias and Semprebon2011). This scale was developed for Equoidea, but because horses have unique traits in their wear patterns, and given that the 0−3 univariate mesowear scale (Rivals et al., Reference Rivals, Mihlbachler and Solounias2007a) was developed for its use in bison, and encompasses most of the possible cusp relief and shape combinations described in nature, we employed the 0−3 scale, transforming the dataset of Danowitz et al. (Reference Danowitz, Hou, Mihlbachler, Hastings and Solounias2016) to this scale. The selected discriminant method was linear and common covariance, because our extended mesowear and mesowear III data came from the same order of magnitude (i.e., scales) and were in covariance (i.e., the same teeth were scored both in the lingual and labial part of the ectoloph). Dietary category (browser, grazer, or mixed feeder) was the grouping variable (Danowitz et al., Reference Danowitz, Hou, Mihlbachler, Hastings and Solounias2016). Finally, to test statistic differences among the multivariate centroids of the dietary categories, we performed a Wilks’ lambda test (α=0.05). The cluster analysis was performed with Statistica 10 software (Statsoft, 2011), whereas the discriminant analysis, chi-square, and Wilks’ lambda tests were carried out with JMP 8.0 (SAS Institute, Inc., https://www.jmp.com).
Repositories and institutional abbreviations
The fossil specimens from La Piedad-Santa Ana and La Cinta-Portalitos are housed at the Colección del Laboratorio de Paleontología, Universidad Michoacana de San Nicolás de Hidalgo (UM, CPOEI, PMB) located at the Faculty of Biology in Morelia, Michoacán, Mexico. Specimens from Viko Vijin are housed at the Colección Científica del Laboratorio de Paleobiología, campus Puerto Escondido, Universidad del Mar (UMPE), Oaxaca, Mexico.
Results
The analyses showed that the mesowear patterns in the Mexican samples of Bison antiquus were similar to those of nonstrict grazers and mixed feeders. A summary of these mesowear patterns is shown in Table 1 (see Supplemental dataset 1 for raw mesowear values). Extended mesowear signatures in both La Cinta-Portalitos and La Piedad-Santa Ana showed a majority of low cusps (66.6% and 54.5 %, respectively), whereas the opposite was found in Viko Vijin, where the total sample displayed high cusps. Rounded cusps were predominant among the samples. On the other hand, mesowear III signatures showed a large proportion of abrasiveness, with indices>2.8, and in some cases, the maximum wear index was reached (see Viko Vijin’s j-junction score, Table 1).
Table 1 Summary of mean mesowear patterns of the fossil Bison antiquus of this study. %high=percentage of high cusps; %low=percentage of low cusps; %sharp=percentage of sharp cusps; %round=percentage of rounded cusps; mesial=mesial enamel band score; distal=distal enamel band score; j-junction=score of the intersection of mesial and distal enamel bands.

The cluster analysis (Fig. 4.1) grouped the three Mexican samples within the nonstrict grazers. Samples from La Cinta-Portalitos and La Piedad-Santa Ana formed one cluster close to the one of Connochaetes taurinus (Burchell, Reference Burchell1824) and Alcelaphus buselaphus (Pallas, Reference Pallas1766). Meanwhile, the Viko Vijin sample formed a cluster with Kobus ellipsiprymnus (Ogilby, Reference Ogilby1833). The univariate mesowear analysis (Fig. 4.2) showed the highest values for La Cinta-Portalitos sample (1.66), followed by La Piedad-Santa Ana sample (1.54), and ending with the Viko Vijin sample (0.93).

Figure 4 Summary of results: (1) hierarchical cluster diagram of mesowear signatures of our samples plus 27 extant model species with known diet; (2) mesowear univariate scores of 50 extant model species with known dietary preferences against Bison antiquus samples from previous studies; (3) mesowear III scores of extant model species and our samples; (4) canonical plot of the discriminant analysis combining extended mesowear univariate signatures with mesowear III signatures. Color code: red=grazers; orange=nonstrict grazers; green=mixed feeders; blue=browsers. AA=Alces alces (Linnaeus, Reference Linnaeus1758); ab=Alcelaphus buselaphus; BaBC=Bison antiquus from Blackwater Draw, Clovis Pit, Curry County, New Mexico; BaBD=B. antiquus from Blackwater Draw, Dry Cave, and Dark Canyon Cave, New Mexico, and Lubbock Lake and Scharbauer Ranch, Texas (Barrón-Ortíz, Reference Barrón-Ortíz2016); BaFQ=B. antiquus from Folsom Quarry, New Mexico (Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b); BaIP=B. antiquus from Ingleside, San Patricio County; BaLC=B. antiquus from La Cinta-Portalitos; BaLP=B. antiquus from La Piedad-Santa Ana; BaPV=B. antiquus from Plainview Quarry, Hale County, Texas (Rivals and Semprebon, Reference Rivals and Semprebon2012); BaVV=B. antiquus from Viko Vijin; Cc=Cervus canadensis (Erxleben, Reference Erxleben1777); cs=Ceratotherium simum (Burchell, Reference Burchell1817); Cs=Capricornis sumatraensis (Bechstein, Reference Bechstein1799); ct=Connochaetes taurinus; DB=Diceros bicornis (Linnaeus, Reference Linnaeus1758); dl=Damaliscus lunatus (Burchell, Reference Burchell1824); DS=Dicerorhinus sumatrensis (Fischer, Reference Fischer1814); eg=Equus grevyi; Et=Eudorcas thomsonii (Günther, Reference Günther1884); eq=Equus quagga Boddaert, Reference Boddaert1785; GC=Giraffa camelopardalis (Linnaeus, Reference Linnaeus1758); he=Hippotragus equinus (Desmarest, Reference Desmarest1804); hn=H. niger (Harris, Reference Harris1838); ke=Kobus ellipsiprymnus; Me=Aepyceros melampus (Lichtenstein, Reference Lichtenstein1812); Ng=Nanger granti (Brooke, Reference Brooke1872); OH=Odocoileus hemionus (Rafinesque, Reference Rafinesque1817); OJ=Okapia johnstoni (Sclater, Reference Sclater1901); Om=Ovibos moschatus (Zimmermann, Reference Zimmermann1780); OV=Odocoileus virginianus (Zimmermann, Reference Zimmermann1780); PBb=plains bison, B. bison; rr=Redunca redunca (Pallas, Reference Pallas1767); RS=Rhinoceros sondaicus (Desmarest, Reference Desmarest1822); To=Taurotragus oryx (Pallas, Reference Pallas1766); Ts=Tragelaphus scriptus (Pallas, Reference Pallas1766); WBb=wood bison, B. bison.
There were no statistically significant differences between the mesowear univariate scores (0−3) of La Cinta-Portalitos and La Piedad-Santa Ana with the grazer guild (p=0.32, 0.1, respectively), but there were statistically significant differences with the mixed feeder (p=0.0002, 0.0003, respectively) and browser guilds (p<0.0001, in both cases). On the other hand, the Viko Vijin scores showed no statistically significant differences with the mixed feeder guild (p=0.29), but statistically significant differences with the grazer (p<0.0001) and browser (p=0.005) guilds.
The mesowear III analysis (Fig. 4.3) showed no statistically significant differences between the scores of La Cinta-Portalitos and the grazer (p=0.54) and mixed feeder (p=0.1) guilds, but there were differences with the browser guild (p<0.0001). As for the scores of La Piedad-Santa Ana, we found no statistically significant differences between the mesowear III scores and the grazer guild (p=0.75), but we found differences with the mixed feeder (p=0.02) and browser (p<0.0001) guilds. The Viko Vijin scores showed no statistically significant differences with the mixed feeder guild (p=0.6), but statistically significant differences with the grazer (p=0.01) and browser (p<0.0001) guilds.
Finally, the discriminant analysis (Fig. 4.4) classified the samples of La Cinta-Portalitos and La Piedad-Santa Ana as grazers with posterior probabilities of 88% and 81%, respectively, whereas the Viko Vijin sample was classified as a mixed feeder, with a probability of 75%. Also, the multivariate centroids of the dietary categories showed statistically significant differences between groups (prob>F ≤ 0.0001).
Discussion
Cluster analysis
In the cluster analysis (Fig. 4.1), the samples from Michoacán-Guanajuato comprised one cluster, and this was close to the one formed by Connochaetes taurinus and Alcelaphus buselaphus, which are identified as nonstrict grazers (Fortelius and Solounias, Reference Fortelius and Solounias2000). The Viko Vijin sample formed a cluster with the waterbuck (Kobus ellipsiprymnus). The blue wildebeest (C. taurinus) diet consisted of 87.5% monocots, 12% dicots, and 0.5% fruits (Gagnon and Chew, Reference Gagnon and Chew2000). The blue wildebeest preferred habitat consisted of open bushland and short grassy plains, always with a close (< 20 km) water source (Kingdon, Reference Kingdon2013). On the other hand, the hartebeest (A. buselaphus) diet comprised 75% monocots, 20% dicots, and 5% fruits (Gagnon and Chew, Reference Gagnon and Chew2000). Hartebeests are considered an ecotone species (Kingdon, Reference Kingdon2013); their preferred habitats are boundaries between open grassy plains (or glades) and parkland, woodland, or scrub; their movements are often within the drainage lines in the dry season and in more open plains during the rainy season (Booth, Reference Booth1985; Kingdon, Reference Kingdon2013). Similarly, Kobus ellipsiprymnus is regarded as a nonstrict grazer (Fortelius and Solounias, Reference Fortelius and Solounias2000); its diet consists of 84% monocots, 15% dicots, and 1% fruits (Gagnon and Chew, Reference Gagnon and Chew2000) and its preferred habitat consists of mosaics of savanna woodlands and forest-savanna, with permanent water sources (Kingdon, Reference Kingdon2013).
The cluster analysis also showed that the modern plains bison (Bison bison) is classified within the strict grazer category (Fortelius and Solounias, Reference Fortelius and Solounias2000), and it is not near the mesowear pattern of our samples of B. antiquus (Fig. 4.1, bb=B. bison). These data suggest a distinct and broader dietary niche for the analyzed ancient Mexican bison populations, when compared to their alleged modern plains analog, and a wider range of habitat use by the Pleistocene B. antiquus individuals analyzed in this study.
Age of studied bison samples
The age of the bison samples used in this study is important for comparisons. The radiometric age of la Piedad-Santa Ana site is unknown, but the age of the other two sites has been inferred. Bison material of Viko Vijin has been dated by the uranium series based on the 230Th-234U-238U method, providing an estimated age of 36 ka for the fossil localities (Ordoñez-Regil et al., Reference Ordoñez-Regil, Almazán-Torres, Jiménez-Hidalgo and Tenorio2016). We inferred the age of La Cinta-Portalitos by the relationship of dated radiocarbon sections of a core made in the Cuitzeo Lake (Israde-Alcántara et al., Reference Israde-Alcántara, Velázquez-Durán, Lozano-García, Bischoff, Domínguez-Vázquez and Garduño-Monroy2010) and the depth in which the fossil bed was found, thus providing an estimated age of 24.05 kyr (with a maximum of ca. 35 kyr and a minimum of ca. 18 kyr). This shows that both northern and southern Mexican samples are of comparable age, both occurring during the middle Wisconsin glacial stage. Under this scenario, caution must be taken when comparing different univariate bison scores from the literature. For example, B. antiquus from New Mexico and New Mexico-Texas samples date back to 10.5 ka (Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b) and 10−15 ka (Barrón-Ortíz, Reference Barrón-Ortíz2016), both close to the Younger Dryas event ca. 10−11.6 ka, and the Bølling-Allerød warm period, respectively (Björck, Reference Björck2007), and far from the estimated ages of our study sites. Other bison samples also have this age pattern; B. priscus from Alaska dates from 11.99 ka (Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b). Only two dated bison samples are known from both glacial (21−60 ka) and late glacial (10−13 ka) stages, unfortunately, these samples were not identified to species level (Barrón-Ortíz, Reference Barrón-Ortíz2016), thus obscuring the paleoecological inference of those mesowear scores because they could represent the signal of several species.
Modern and extinct bison wear patterns
Our results suggest that the Mexican samples analyzed display a mixed feeder pattern, rather than a strict grazer pattern. This is like the diet of Bison antiquus from Rancho La Brea, inferred from dental boluses in which the consumption of grasses was only 13.4% of the diet, compared to 86.6% gymnosperms and dicotyledons (Akersten et al., Reference Akersten, Foppe and Jefferson1988).
On the other hand, our extended mesowear univariate scores show two patterns: more abrasive scores for La Cinta-Portalitos (1.66) and La Piedad-Santa Ana (1.54), and a less abrasive score for Viko Vijin (0.93). The scores from the northern Mexican samples (Fig. 4.2) are similar to one previously known Bison antiquus score (1.56) from Plainview Quarry (BaPV), Hale County, Texas (Rivals and Semprebon, Reference Rivals and Semprebon2012), and are lower than the 1.83 score from Ingleside (BaIP), San Patricio County, Texas (Rivals and Semprebon, Reference Rivals and Semprebon2012), the 1.93 score from Blackwater Draw (BaBC), Clovis Pit, Curry County, New Mexico (Rivals and Semprebon, Reference Rivals and Semprebon2012), and the 1.88 score from Blackwater Draw (BaBD), Dry Cave, and Dark Canyon Cave, New Mexico and Lubbock Lake, and Scharbauer Ranch, Texas (Barrón-Ortíz, Reference Barrón-Ortíz2016). Also, our scores from northern Mexico are higher than the 1.31 score from Folsom Quarry, Union County, New Mexico (Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b). In contrast, the Viko Vijin sample score is the lowest for the species (0.93). The low latitude provenance of the sample and the association of the diet and the different environment and vegetation structure can explain this low score, as was noted above.
Mesowear univariate scores from other bison fossil species are known. Two samples of Bison sp. from Dalhart Sideroad Pit, Channing, Hartley County, Texas, and Seminole Field Station B, Pinellas County, Florida display a 1.05 mesowear score (Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b). There are two known Bison sp. fossil samples from Alberta, Canada, with mesowear data at two different times, one preglacial sample with a 1.67 score, and one postglacial sample with a 1.86 score (Barrón-Ortíz, Reference Barrón-Ortíz2016). A sample of the steppe bison (B. priscus) from the Fairbanks area, Alaska, displays a score of 1.1 (Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b). Despite this mesowear spectrum, the genus Bison and its fossil species still all fall within the grazer univariate score range (Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b). The northern Mexican samples display the highest values, and the Viko Vijin sample has the lowest values. The differences between the Mexican samples are probably due to dissimilarities in the diet and habitat use between these more middle- to high-latitude species and our sample individuals.
The univariate wear pattern of modern Bison bison ecotypes is also different from our sample patterns. Modern plains bison (B. bison bison), grouped with the strict grazer guild in the cluster analysis (Fig. 4.1), display a very high mesowear score (2.73), the highest value among the extant model species (Fortelius and Solounias, Reference Fortelius and Solounias2000; Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b). On the other hand, the wood bison (B. bison ‘athabascae’ Rhoads, Reference Rhoads1897) grouped within the mixed feeder guild in the cluster analysis (Fig. 4.1), and displays a mesowear score of 1.0, which is outside the range of typical grazers (Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b). We consider these modern scores as two different ecological behaviors of the same species because the modern status of wood bison as a separate subspecies is dubious (Geist, Reference Geist1991; Groves and Grubb, Reference Groves and Grubb2011). The modern bison and its ecotypes display a wide range of variation among its wear signals. Our samples display a similar wear spectrum, and this might also reflect notable differences between the diets of our northern and southern Mexican samples and niche plasticity as wide as that described for modern bison. The Viko Vijin sample has the lowest wear values of all known bison (extant and extinct), although it is very similar to the pattern of modern wood bison (Fig. 4.2). This could reflect a similar composition in the abrasiveness in the diet of modern wood bison and extinct B. antiquus from Viko Vijin, as well as important differences between the feeding behavior of northern Mexican samples of B. antiquus and modern plains bison, making the latter an unsuitable ecological analog for B. antiquus, because its diet is apparently more abrasive and restricted to grasses. This is supported by the analysis of dental boluses of B. antiquus from California (Akersten et al., Reference Akersten, Foppe and Jefferson1988).
Dietary geographical variation and grit consumption
A cline of wear (Fig. 4.2, 4.3) of the studied samples (both for extended mesowear and mesowear III) is apparent and seems to be associated with latitude (i.e., the northern samples show more wear in their teeth than the southern ones). This can be explained by differences in the vegetation structure and the diet of the individuals (Rivals et al., Reference Rivals, Solounias and Mihlbachler2007b) or by the presence of exogenous materials ingested by the northern bison individuals (Damuth and Janis, Reference Damuth and Janis2011; Hoffman et al., Reference Hoffman, Fraser and Clementz2015). There is some evidence against dust, quartz sand, and other exogenous materials as the main source of the wear patterns observed. For example, Antilocapra americana (Ord, Reference Ord1815) inhabits highly dusty and open environments and its mesowear signature contains a very high proportion (88.6%) of sharp cusps (Fortelius and Solounias, Reference Fortelius and Solounias2000). Also, there is an experimental approach that shows the importance of vegetal endogenous SiO2 particles in the abrasion of enamel, pointing out that wear is mainly caused by the mechanical forces involving chewing of the vegetal material rather than just ingesting exogenous grit (Xia et al., Reference Xia, Zheng, Huang, Tian, Chen, Zhou, Ungar and Qian2015). However, we cannot completely discard that volcanic ash, dust, and other quartz-related exogenous materials were involved to some degree in shaping of the observed wear patterns. Also, these exogenous particles are more abundant in open areas (Janis and Fortelius, Reference Janis and Fortelius1988; Fortelius and Solounias, Reference Fortelius and Solounias2000), such as the ones previously inferred for the fossil localities of the northern samples (Gutiérrez Bedolla et al., Reference Gutiérrez Bedolla, García-Zepeda, López-García, Arroyo-Cabrales, Marín-Leyva, Meléndez-Herrera and Fuentes-Farías2016; Marín-Leyva et al., Reference Marín-Leyva, DeMiguel, García-Zepeda, Ponce-Saavedra, Arroyo-Cabrales, Schaaf and Alberdi2016b), which supports the ingestion of grasses. Given that the studied sites have similar elevations—La Cinta-Portalitos ranges from 1750−2350 masl (meters above sea level), La Piedad-Santa Ana ranges from 1700−2500 masl, and Viko Vijin ranges from 1800−2400 masl (Jiménez-Hidalgo et al., Reference Jiménez-Hidalgo, Guerrero-Arenas, Macfadden and Cabrera-Pérez2011; Díaz-Sibaja, Reference Díaz-Sibaja2013)—altitude seems not to be related to the observed dietary trends.
Mesowear and previous paleoenvironmental reconstructions of localities
Our data support and expand on previous paleoenvironmental scenarios for La Cinta-Portalitos and La Piedad-Santa Ana. The evidence suggests the main presence of open areas with a dominance of grasses. All in agreement with isotopic (δ13C and δ18O) and microwear data from Equus spp. and Mammuthus columbi (Falconer, Reference Falconer1858) (Gutiérrez Bedolla et al., Reference Gutiérrez Bedolla, García-Zepeda, López-García, Arroyo-Cabrales, Marín-Leyva, Meléndez-Herrera and Fuentes-Farías2016; Marín-Leyva et al., Reference Marín-Leyva, Arroyo-Cabrales, García-Zepeda, Ponce-Saavedra, Schaaf, Pérez-Crespo, Morales-Puente, Cienfuegos-Alvarado and Alberdi2016a), mesowear data from horses (Marín-Leyva et al., Reference Marín-Leyva, Arroyo-Cabrales, García-Zepeda, Ponce-Saavedra, Schaaf, Pérez-Crespo, Morales-Puente, Cienfuegos-Alvarado and Alberdi2016a), palynological data (Israde-Alcántara et al., Reference Israde-Alcántara, Velázquez-Durán, Lozano-García, Bischoff, Domínguez-Vázquez and Garduño-Monroy2010), and the scarcity of typical closed-habitat mammals in these paleocommunities such as tapirs. Our mesowear bison data expand this scenario and suggest the presence of a more heterogeneous vegetation structure, mainly for La Cinta-Portalitos, where previous palynological data point to the presence of other botanical elements such as Cheno-Am, Ambrosia spp., Asteraceae indet., Cirsium spp., and Thalictrum spp. during the time interval in which bison inhabited the area (Israde-Alcántara et al., Reference Israde-Alcántara, Velázquez-Durán, Lozano-García, Bischoff, Domínguez-Vázquez and Garduño-Monroy2010), and several typical mixed feeder mammals such as pronghorns and camelids (Díaz-Sibaja et al., 2014a, Reference Díaz-Sibaja, Ponce-Saavedra, Arroyo-Cabrales, Jiménez-Hidalgo and García-Zepedab; Plata-Ramírez et al., Reference Plata-Ramírez, García-Zepeda, Arroyo-Cabrales and Marín-Leyva2015).
Our mesowear data also support previous paleoenvironmental scenarios for Viko Vijin local fauna. The bison samples of this fauna come from localities Oax-3 La Pedrera and Oax-7 Río Tejupam. The inferred paleoenvironment for Oax-3 is a floodplain (Jiménez-Hidalgo et al., Reference Jiménez-Hidalgo, Guerrero-Arenas, Macfadden and Cabrera-Pérez2011) with the presence of short-lived vegetation in a wetland setting (Guerrero-Arenas et al., Reference Guerrero-Arenas, Jiménez-Hidalgo and García-Barrera2013). Locality Oax-7 was fluvial, possibly a meandering system in an open setting (Jiménez-Hidalgo et al., Reference Jiménez-Hidalgo, Guerrero-Arenas, Macfadden and Cabrera-Pérez2011). Our mesowear data support these environmental settings and suggest a more heterogeneous vegetation structure, with mosaic vegetation, and little dominance of grasses. This is supported by the presence of typical open habitat mammals such as Equus spp. and Mammuthus spp., as well as typically closed habitat mammals such as Odocoileus spp. and Cuvieronius spp., coexisting with some genera identified as mixed feeders such as Hemiauchenia spp. and Camelops spp. (Jiménez-Hidalgo et al., Reference Jiménez-Hidalgo, Guerrero-Arenas, Macfadden and Cabrera-Pérez2011).
Conclusions
The combined mesowear analysis showed that our two northern Mexican samples of Bison antiquus fall within the grazer guild and the southern one falls within the mixed feeder guild. These mesowear signatures are different from those of most of the previously reported samples of B. antiquus, and from modern B. bison. The Viko Vijin mesowear score is close to that of the wood bison (B. bison ‘athabascae’) suggesting similar dietary behavior. These mesowear patterns show broader dietary habits for B. antiquus and a low abrasive ingestion in the southernmost populations. These interpretations fit within a more generalist diet than previously assumed for this species.
The difference among mesowear scores is related to latitude, reflecting different environmental conditions. Based on previous evidence (i.e., isotopic, microwear, mesowear, palynological, and sedimentological data, as well as the composition of vertebrate and invertebrate-associated fauna), we agree that the Mexican studied sites were open areas where the grasses were nondominant and probably a more heterogeneous landscape. These paleoenvironments were coetaneous, occurring during the early and middle Wisconsin glacial stages.
Our paleoecological inferences on the diet of these bison samples require further testing. Future studies must be focused on other species found in these fossil mammal assemblages, and in other proxies such as microwear and stable carbon isotope analyses.
Acknowledgments
We wish to thank all of the members of the Paleontology Laboratory (Laboratorio de Paleontología) from the UMSNH (Universidad Michoacana de San Nicolás de Hidalgo) and the OEI (Organización Especial de Investigación de La Piedad) who participated in the field campaigns of the fossiliferous deposits of Michoacán-Guanajuato and kindly allowed us to study their collections. We thank the kind help of F. Rivals for providing the raw mesowear data of the fossil bison from Blackwater Draw, New Mexico and for clarifying the use of the 0−3 univariate mesowear score. We acknowledge the comments of two anonymous reviewers, which helped improve this paper. Funding for collecting the Pleistocene specimens from Viko Vijin was provided by CONACyT-Ciencia Básica project 101626 to EJ-H.
Accessibility of supplemental data
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.9k4h503.







