Introduction
Anacardiaceae Brown, Reference Brown and Tuckey1818, nom. cons., is a family of cosmopolitan distribution composed of evergreen or deciduous shrubs and trees with secretory canals in the leaves, bark, and, often, wood, mainly distributed in pantropical regions, with some elements found in temperate regions of Eurasia and South America (Muñoz, Reference Muñoz and Hunziker2000). The family includes ~80 genera and nearly 880 species (Stevens, Reference Stevens2001–onwards). In Argentina, there are 33 extant species in six genera (Astronium Jacq., Lithraea Hook. and Arn., Mauria Kunth, Schinopsis Engl., Schinus L., Loxopterygium Hook. F.), six of which are endemic species of Schinus (Zuloaga and Morrone, Reference Zuloaga and Morrone1999; Muñoz, Reference Muñoz and Hunziker2000).
Anacardiaceous fossil palynomorphs and leaf impressions with affinities to Lithraea Miers., Schinus, and Astronium were recovered in the Miocene Paraná and Ituzaingó formations from northeastern Argentina (Anzótegui, Reference Anzótegui1990; Anzótegui and Aceñolaza, Reference Anzótegui and Aceñolaza2008; Franco et al., Reference Franco, Brea, Orfeo, Zucol, Brandoni and Noriega2013). Recently, a fossil leaf type from the middle Miocene of Patagonia was described and assigned to the Anacardiaceae and shows a great similarity to Lithraea molleodes (Vellozo) Engler, Reference Engler1876 (Passalia et al., Reference Passalia, Caviglia and Vera2019).
Moreover, in the last ten years, the number of anacardiaceous fossil woods found in the late Cenozoic of northeastern Argentina has increased significantly (Franco and Brea, Reference Franco and Brea2008; Franco, Reference Franco2009, Reference Franco2011; Brea et al., Reference Brea, Zucol and Patterer2010), with 17 taxa now reported (Table 1). Some Argentinean fossil woods resemble the extant genera Astronium, Schinopsis, and Schinus (Brea and Franco, Reference Brea and Franco2013). Among them, Schinopsixylon was proposed by Lutz (Reference Lutz1979) and contains two species: S. herbstii Lutz, Reference Lutz1979 and S. heckii Lutz, Reference Lutz1979 (Lutz, Reference Lutz1979; Brea, Reference Brea1999; Brea et al., Reference Brea, Zucol and Patterer2010; Franco, Reference Franco2011). Astroniumxylon was erected by Brea et al. (Reference Brea, Aceñolaza and Zucol2001) and contains three species: A. portmanii Brea, Aceñolaza, and Zucol, Reference Brea, Zucol, Albert and Reis2001; A. parabalansae Franco and Brea, Reference Franco and Brea2008; and A. bonplandianum Franco, Reference Franco2009 (Brea et al., Reference Brea, Aceñolaza and Zucol2001; Franco and Brea, Reference Franco and Brea2008; Franco, Reference Franco2009, Reference Franco2011). In addition, Resinaxylon Pujana, an anacardiaceaous fossil wood resembling Schinus, was found in the Oligocene San Julián and Río Leona Formations (Patagonia, Argentina; Pujana, Reference Pujana2009; Martínez and Pujana, Reference Martínez and Pujana2010).
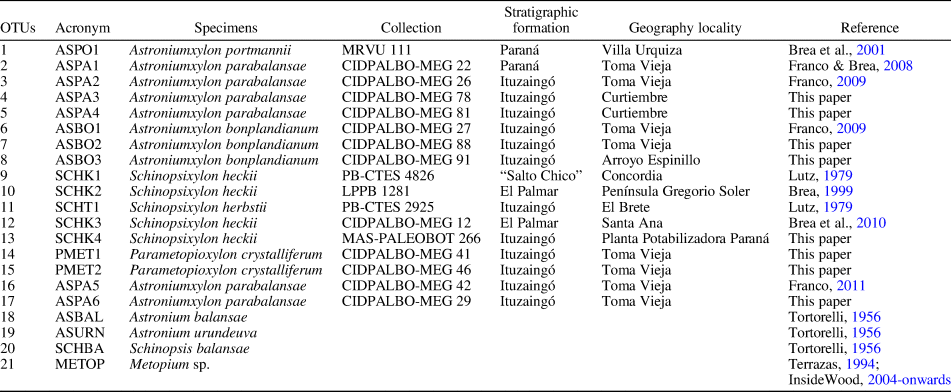
Table 1. List of OTUs selected for the multivariate analysis.
Some salient secondary xylem features of the Anacardiaceae are: simple perforation plates (except in some species with both simple and scalariform plates); alternate intervessel pits; parenchyma paratracheal, scanty, vasicentric, or aliform, with marginal bands in some genera; rays mostly 2–3 cells wide, but larger in some species, usually heterocellular; fibers with simple pits, sometimes septate; and intercellular canals in lots of genera (Metcalfe and Chalk, Reference Metcalfe and Chalk1950; Terrazas, Reference Terrazas1999; Giménez et al., Reference Giménez, Calatayu, Diaz Zirpolo, Figueroa and Gonzalez2015). The anatomical structure of the Anacardiaceae xylem is fairly homogeneous (Heimsch, Reference Heimsch1942; Agarwal and Gupta, Reference Agarwal and Gupta2008; Gupta and Agarwal, Reference Gupta and Agarwal2008; Schweingruber et al., Reference Schweingruber, Börner and Schulze2011), but some features have been proposed to differentiate genera from each other (Heimsch, Reference Heimsch1942; Terrazas, Reference Terrazas1994). Because multivariate analysis of comparative wood anatomy has been shown to be helpful in distinguishing species or groups of species in extant and fossil genera, such as in the Anacardiaceae, Nothofagaceae, Leguminosae, and Podocarpaceae (Terrazas and Wendt, Reference Terrazas and Wendt1995; Poole, Reference Poole2002; Pujana et al., Reference Pujana, Martínez, García Massini, Di Orio and Penas Steinhardt2014a, Reference Pujana, Santillana and Marenssib), we conducted a similar analysis to clarify the systematics of Anacardiaceae fossil woods recovered in southern South America.
The aims of this study are: (1) to make a taxonomic revision of the late Cenozoic Anacardiaceae fossil woods from northeastern Argentina; (2) to describe eight new silicified woods assigned to four different species of Anacardiaceae; (3) to determine anatomical variability and similarities among fossil Anacardiaceae woods found in the Ituzaingó, Paraná, El Palmar, and “Salto Chico” formations, and related extant genera using correspondence and cluster analyses to establish the taxonomic limits and the diagnostic characters in fossil taxa; and (4) to propose a key to distinguish species of Parametopioxylon new genus, Schinopsixylon, and Astroniumxylon from each other.
Materials and methods
Eight new wood specimens are described, all of which were collected from the Ituzaingó Formation at the Toma Vieja, Curtiembre and Arroyo El Espinillo localities (Fig. 1). These fossil woods are silicified and were thin-sectioned in transverse, tangential, and radial planes following standard techniques for petrified woods. The specimens are registered in the “Colección Paleobotánica del Laboratorio de Paleobotánica, CICYTTP (CONICET-Prov. ER-UADER)” at Diamante city under the acronyms CIDPALBO-MEG for the fossil woods and CIDPALBO-MIC for the slides, and in the “Museo de Ciencias Naturales y Antropológicas Profesor Antonio Serrano” at Paraná city under the acronym MAS-PALEOBOT, Entre Ríos Province, Argentina. These sections were studied with a Nikon Eclipse E200 light microscope and photomicrographs were taken with a Nikon Coolpix S4 digital camera. For quantitative data, at least 25 measurements were taken. The mean values are shown, followed by minimum and maximum, in the description of each specimen. In addition, small fragments of wood were studied with a scanning electron microscope (SEM). The material was prepared for SEM by cutting a 1 cm3 block of wood, mounting it on SEM stubs without coating, and then observing under low vacuum using a Phenom Pro Desktop SEM scanning electron microscope at the Electron Microscope Laboratory (Laboratorio de Microscopía Electrónica-EMLAB “Dr. Domingo Liotta”), CICYTTP (CONICET-Prov. ER-UADER), Diamante, Entre Ríos, Argentina.
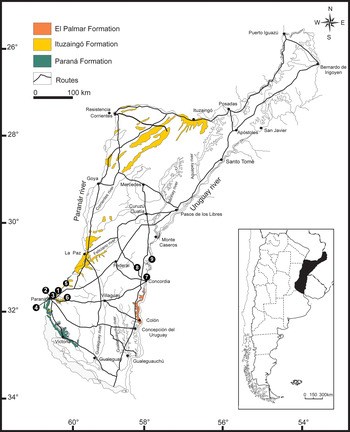
Figure 1. Geological map of the Mesopotamia Argentina showing the geological units (El Palmar, Ituzaingó, and Paraná formations) modified from Brea and Zucol (Reference Brea, Zucol, Albert and Reis2011) and the fossil species localities: (1) Villa Urquiza, (2) Toma Vieja, (3) El Brete, (4) Planta Potabilizadora, (5) Curtiembre, (6) Arroyo El Espinillo, (7) Concordia, (8) Península Gregorio Soler, and (9) Santa Ana.
The descriptions of woods given below conform to the IAWA Hardwood List (IAWA Committee, 1989). For comparison with extant species, we used the InsideWood website and its coding (InsideWood, 2004–onwards; Wheeler, Reference Wheeler2011) and references to classical descriptions of extant and fossil plants (Heimsch, Reference Heimsch1942; Wagemann, Reference Wagemann1948; Metcalfe and Chalk, Reference Metcalfe and Chalk1950; Tortorelli, Reference Tortorelli1956; Terrazas, Reference Terrazas1994, Reference Terrazas1999; Terrazas and Wendt, Reference Terrazas and Wendt1995; Agarwal and Gupta, Reference Agarwal and Gupta2008; Gupta and Agarwal, Reference Gupta and Agarwal2008, Giménez et al., Reference Giménez, Calatayu, Diaz Zirpolo, Figueroa and Gonzalez2015). The InsideWood database provides coded wood anatomical descriptions based on the IAWA List of Microscopic Features (i.e., each anatomical feature has a number code). Most features are qualitative (showing presence [p] or absence [a]), but some are quantitative (Wheeler, Reference Wheeler2011).
The systematic assignment follows the APG IV (2016). Relationships to extant and fossil groups were investigated by using the references found in bibliographic lists made by Gregory (Reference Gregory1994), Gregory et al. (Reference Gregory, Poole and Wheeler2009), and Kew Micromorphology Bibliographic web site (https://www.kew.org/epic/datasources.htm). With respect to the nomenclature used in the new fossil woods, the specimens were assigned to fossil rather than modern genera because: (1) the fossils are represented only by an isolated organ for which the remainder of the plant is unknown, and (2) there is a convention to match taxonomic rank with geological time. In this case, the fossils were found in the Ituzaingó Formation (late Miocene?) and it has been proposed to use modern generic names from the Pliocene (Collinson, Reference Collinson, Spicer and Thomas1986; Avise and Johns, Reference Avise and Johns1999). This protocol allowed us to unify criteria with previous work and facilitated the comparison of the material with other fossils.
In addition, a taxonomic revision based on 17 fossil specimens (10 Astroniumxylon, five Schinopsixylon, and two Parametopioxylon n. gen., including type species of each) and four extant species (Astronium balansae Engler, Reference Engler1881; Astronium urundeuva (Allemão) Engler, Reference Engler1881; Schinopsis balansae Engler, Reference Engler1885; and Metopium sp.) was performed. The specimens examined are shown in Table 1.
The anatomical characters used in this study were considered in previous taxonomic papers to analyze phylogenetic and ecological aspects of the Anacardiaceae (Terrazas, Reference Terrazas1994) and historical biogeography of Anacardiaceae based on fossil wood anatomy (Martínez-Millán, Reference Martínez Millán2000). We used the characters that Terrazas (Reference Terrazas1994) and Martínez-Millán (Reference Martínez Millán2000) found useful for distinguishing Anacardiaceae species (Supplementary Material 1), to which we added the percentage of uniseriate, biseriate, and multiseriate rays.
Our complete Data Matrix (DM) consist of 21 binary and 12 multi-state characters, using the criteria of Crisci and López Armengol (Reference Crisci and López Armengol1983). The characters used in the multivariate analysis with their corresponding states and codes and the DM are shown in Supplementary Material 1 and 2. If the fossil material was too badly preserved to identify the value of a character, the character was coded as “not comparable,” and a question mark was used in the DM (Supplementary Material 2). Therefore, these character states were not included in the calculation process. The multivariate analyses were performed on a final DM that includes 22 wood anatomical characters because 11 were excluded either because they do not show variability, they are present in only one OTU, or the character can be highly influenced by environmental conditions (Baas and Wheeler, Reference Baas, Wheeler, Hodkinson, Jones, Waldren and Parnell2011, and references herein).
A Correspondence Analysis (CA) was performed to summarize similarities of anatomical variability among fossil specimens, and corroborate the accuracy of the systematic placement. Also, groups were classified by cluster analysis constructed using the UPGMA algorithm and based on Gower's distance matrix among OTUs. This coefficient can be used with a mixture of character types (binary, qualitative, and quantitative) as well as allowing missing values. All multivariate analyses were performed with the PAST software package version 2.17c (Hammer et al., Reference Hammer, Harper and Ryan2001).
Repositories and institutional abbreviations
Types and other specimens examined in this study are deposited in the following institutions and repositories: Museo Regional de Villa Urquiza (MRVU), Entre Ríos, Argentina; Colección Paleobotánica del Laboratorio de Paleobotánica, Centro de Investigación Científica y de Transferencia Tecnológica a la Producción-CICYTTP (CONICET-Prov. ER-UADER) (CIDPALBO-MEG and CIDPALBO-MIC), Diamante, Entre Ríos, Argentina; Colección Paleobotánica “Dr. Rafael Herbts” (PB-CTES), Facultad de Ciencias Exactas y Naturales y Agrimensura, Universidad Nacional del Nordeste, Corrientes, Argentina; Colección Paleobotánica, Museo de La Plata (LPPB), La Plata, Argentina; Museo de Ciencias Naturales y Antropológicas “Profesor Antonio Serrano” (MAS-PALEOBOT), Paraná, Entre Ríos, Argentina.
Systematic paleobotany
All the specimens studied in this paper show diagnostic features of the Anacardiaceae: diffuse to semi-ring-porous, tyloses present, solitary, radial multiples, and in cluster vessels, simple perforation plates, alternate to opposite intervessel pits, parenchyma and ray-vessel pits with much reduced borders to apparently simple; pits horizontal or similar to intervessel pits in size and shape throughout the ray cells, predominantly paratracheal vasicentric parenchyma, heterocellular rays, presence of radial canals in multiseriate rays and prismatic crystals common (Metcalfe and Chalk, Reference Metcalfe and Chalk1950; Terrazas, Reference Terrazas1994, Reference Terrazas1999; Gupta and Agarwal, Reference Gupta and Agarwal2008; Giménez et al., Reference Giménez, Calatayu, Diaz Zirpolo, Figueroa and Gonzalez2015).
Order Sapindales Berchtold and Presl, Reference Berchtold and Presl1820
Family Anacardiaceae Brown, Reference Brown and Tuckey1818, nom. cons.
Genus Parametopioxylon new genus
Type species
Parametopioxylon crystalliferum n. gen. n. sp., by monotypy.
Diagnosis
As for type species.
Occurrence
Ituzaingó Formation (late Miocene? sensu Brunetto et al., Reference Brunetto, Noriega, Brandoni, Brandoni and Noriega2013), outcropping at Toma Vieja, Entre Ríos Province, Argentina.
Etymology
The generic name denotes the wood's resemblance to extant Metopium Browne, Reference Browne1756.
Parametopioxylon crystalliferum new species
Figures 2–4

Figure 2. Parametopioxylon crystalliferum n. gen. n. sp., holotype CIDPALBO-MEG 41. (1) transverse section (TS) showing growth rings distinctly demarcated by marginal parenchyma (arrows), vessels, and parenchyma; (2) radial longitudinal section (RLS) showing a radial canal; (3, 4) tangential longitudinal sections (LTS) showing rays and vessels; note the numerous radial canals in multiseriate rays; (5) TS with solitary vessels, vasicentric parenchyma, and rays; note the radial canal in a multiseriate ray; (6) TS showing vessels with tyloses, vasicentric, and confluent parenchyma, and rays; note prismatic crystals in ray cells; (7) detail of canals in multiseriate rays; (8) heterocellular rays with prismatic crystals in longitudinal radial section; (9) TS showing vessel with aliform parenchyma. Scale bars are (1) 500 μm; (2–8) 400 μm; (9) 100 μm.

Figure 3. Parametopioxylon crystalliferum n. gen. n. sp., holotype CIDPALBO-MEG 41 (2), (4–13) and paratype CIDPALBO-MEG 46 (1), (3). (1, 2) Vessels in cluster (arrow); (3) fibers in TS, the arrow indicates marginal parenchyma; (4) detail of a heterocellular ray in LRS, the black arrows indicate prismatic crystals in upright cells and the white arrow in procumbent cells; (5) parenchyma and ray-vessel pitting horizontal (white arrow) and similar to intervessel pits in size and shape throughout the parenchyma cells (black arrow); (6) detail of a radial canal, in LTS; (7–9) uniseriate and biseriate rays (black arrows); (10) prismatic crystal in upright ray cell (black arrow); (11) prismatic crystals in procumbent ray cell (black arrows); (12) ray-vessel pitting similar to intervessel pits (black arrow); (13) parenchyma-ray pitting with much-reduced borders to apparently simple: pits horizontal. Scale bars are (1–6) 50 μm; (7–13) 20 μm.

Figure 4. Parametopioxylon crystalliferum n. gen. n. sp. (1) LTS showing rays (white arrows) and vessels; (2), LTS showing multiseriate rays with radial canals (black arrow) and a vessel; (3) detail of a radial canal (black arrow); (4, 6) detail of bordered and alternate to opposite intervessel pits (arrows) (5) simple perforation plate (black arrow) and alternate to opposite intervessel pits (white arrow) LTS. (7) parenchyma strand, parenchyma vessel pitting with much reduced border (black arrow) and fibers with minutely simple pits (white arrow); (8, 9) detail of horizontal parenchyma vessel pitting (arrows); (10) vessel with tyloses and simple perforation plate (black arrow); (11) detail of a heterocellular ray; (12), prismatic crystals in procumbent cells. Scale bars are (1, 2) 100 μm, (3, 11) 30 μm, (4, 7, 8) 50 μm, (5, 10) 80 μm, and (6, 9, 12) 10 μm.
Holotype
CIDPALBO-MEG 41, CIDPALBO-MIC 691 from the Ituzaingó Formation, Toma Vieja, Entre Ríos Province, Argentina.
Paratypes
CIDPALBO-MEG 46, CIDPALBO-MIC 696.
Diagnosis
Diffuse to semi-ring-porous; vessels without arrangement or in diagonal pattern, solitary, in radial multiples of 2–3 (4–5) or in clusters; vessel elements moderately numerous (>20 per mm2) and narrow; very small to small, bordered, and alternate to opposite intervessel pitting; abundant tyloses; parenchyma and ray-vessel pitting with much-reduced borders to apparently simple (pits horizontal) or similar in size and shape to intervessel pits; exclusively simple perforation plates; rays mostly 1–3 seriate, heterocellular rays composed of procumbent and upright cells; some rays with one or more canals and >500 μm in height; fibers non-septate with simple bordered pits; axial parenchyma paratracheal predominantly vasicentric, sometimes confluent, aliform and apotracheal marginal; prismatic crystals mainly in chambered upright and/or square ray cells, and occasionally in procumbent cells.
Occurrence
Ituzaingó Formation (late Miocene?), outcropping at Toma Vieja, Entre Ríos Province, Argentina.
Description
The fossil woods are in a very good state of preservation; the holotype has a diameter of 14 cm and is 13.5 cm long, and the paratype has a diameter of 16 cm and is 31 cm long. Growth rings are distinct, demarcated by marginal parenchyma (Figs. 2.1, 3.1). Vessels are diffuse to semi-ring-porous (Fig. 2.1). Vessels are randomly arranged or with a tendency to diagonal pattern (Fig. 2.1). Vessels are circular or oval in cross-section with frequent tyloses and other deposits (Figs. 2.1, 2.5, 2.6, 4.10). Some crystals are in tyloses (Fig. 4.10). Vessels are mainly solitary (45%), in radial multiples of two (25%), three (10%), four (6%), five (3%), or more (2%), and in clusters (9%) (Figs. 2.1, 2.5, 2.6, 3.1, 3.2). The mean vessel tangential diameter is 94 (34–127) μm, and the mean vessel radial diameter is 108 (25–167) μm. There are, on average, 26 (19–31) pores per mm2. The mean vessel element length is 237 (167–333) μm. The mean vessel wall thickness is 8 (3–13) μm. Vessel elements have straight to oblique end walls (Fig. 4.4). Perforation plates are simple (Fig. 4.5, 4.10). Intervessel pitting has an average diameter of 6 (2–8) μm, alternately to oppositely arranged, bordered with large lenticular apertures, and circular to oval in outline (Fig. 4.4–4.6). Parenchyma and ray-vessel pitting have much-reduced borders to apparently simple pits horizontal or are like intervessel pits in size and shape throughout the ray cells (Figs. 3.5, 3.12, 3.13, 4.7–8). Fibers are non-septate, rounded to polygonal in outline and arranged in radial rows (Fig. 3.3). Fibers are thin- to thick-walled; the mean fiber diameter is 13 (7–18) μm and the walls are 5 (3–6) μm thick. Minute simple pits are abundant, confined to radial walls (Fig. 4.7). Axial parenchyma is paratracheal, mostly vasicentric, sometimes confluent, lozenge-aliform and apotracheal marginal (Fig. 2.1, 2.5, 2.6, 2.9); 2–7 cells per parenchyma strand (Fig. 4.7). Rays are heterocellular. Body ray cells either are procumbent (3–30 rows of cells) with 1–4 rows of upright and/or square marginal cells, or are procumbent, square and upright mixed throughout the ray (Figs. 2.2, 2.8, 3.4, 4.11). Rays are uniseriate (23%), biseriate (35%), triseriate (24%), tetraseriate (6%), and wider with canals (12%) (Figs. 2.3, 2.4, 3.6–3.9, 4.1, 4.2). There are 11 (7–15) rays per linear mm. They are 204 (70–500) μm high, 21 (10–40) μm wide, 46 (24–79) cells high, and 9 (6–12) cells wide (Fig. 2.3, 2.4). Some multiseriate rays have numerous canals (Figs. 2.4, 2.7, 3.6, 4.2, 4.3); rays with canals are sometimes >1 mm high. Canals have a mean diameter of 56 (25–80) μm. Mineral inclusions in the form of prismatic crystals are mainly in chambered upright and/or square ray cells, and occasionally in procumbent cells, and in tyloses (Figs. 2.8, 3.4, 3.10, 3.11, 4.12).
Etymology
The specific name, crystalliferum, refers to the abundance of crystals in the wood.
Remarks
The genus Parametopioxylon n. gen. resembles Metopium (see Terrazas, Reference Terrazas1994; InsideWood, 2004–onwards). It was compared with fossil genera assigned to Anacardiaceae, but shows distinct diagnostic features: diffuse to semi-ring-porous, vessels without arrangement or in a somewhat diagonal pattern, abundant tyloses, simple perforation plates, alternate-to-opposite intervessel pitting, heterocellular rays, some multiseriate rays with canals and reaching a height of >500 μm, axial parenchyma paratracheal predominantly vasicentric, sometimes confluent, aliform, and apotracheal marginal, prismatic crystals in upright/square and procumbent ray cells.
When our material is scored for the presence of growth rings (1p), wood diffuse-porous (5p), simple perforation plates (13p), alternate intervessel pits (22p), tyloses (56p), gums and other deposits in heartwood vessels (58p), simple to minutely bordered pits in fibers (61p), vasicentric (79p), axial parenchyma in marginal or in seemingly marginal bands (89p), rays width 1–3 wide (97p), radial canals (130p), prismatic crystals (136p) in upright or square (137p) and procumbent ray cells (138p) and absence of vessels in tangential bands (6a) or in dendritic pattern (8a), vessels exclusively solitary (9a), vessels in radial multiples of 4 or more (10a), vessel clusters common (11a), scalariform perforation plates (14a), large intervessel pit (27a), axial parenchyma diffuse-in-aggregates (77a) or in bands more than three cells wide (85a) and ray height >1 mm (102a), a search of the InsideWood database (2004–onward) returns Metopium sp. as the only modern wood that matches these characters. There are two features that are “variable” both in Parametopioxylon crystalliferum n. gen. n. sp. and in the extant genus Metopium: wood semi-ring-porous (4p) and vessels in diagonal and/or radial pattern (7p).
Genus Astroniumxylon Brea, Aceñolaza, and Zucol, Reference Brea, Aceñolaza and Zucol2001
Type species
Astroniumxylon portmannii Brea, Aceñolaza, and Zucol, Reference Brea, Aceñolaza and Zucol2001 by original designation. Paraná Formation (late Miocene), Villa Urquiza, Entre Ríos Province, Argentina.
Astroniumxylon bonplandianum Franco, Reference Franco2009
Figure 5.1, 5.2

Figure 5. (1, 2) Astroniumxylon bonplandianum Franco, Reference Franco2009, CIDPALBO-MEG 91: (1) Cross section showing vessels with tyloses, parenchyma paratracheal, and fibers in files radially oriented; (2) longitudinal tangential section showing linear rays mainly uniseriate and biseriate, and multiseriate ray with radial canal (arrow). (3–5) Astroniumxylon parabalansae Franco et Brea, Reference Franco and Brea2008: (3) CIDPALBO-MEG 78, cross section showing vessels with tyloses and distinct growth ring (arrow); (4) CIDPALBO-MEG 81, longitudinal section showing rays mostly 1–3 seriate, multiseriate ray with radial canal (black arrow) and prismatic crystals in ray cells (white arrow); (5) CIDPALBO-MEG 81, longitudinal radial section showing heterocellular rays with one or two rows of square marginal cells, the arrow indicates a prismatic crystal. (6–12) Schinopsixylon herbstii Lutz, Reference Lutz1979 emended, CIDPALBO-MEG 70. (6) Cross section showing diffuse porosity and vessels with tyloses; (7) detail of vessels and scanty axial parenchyma in cross section; (8) general view of longitudinal radial section, the arrow indicates a simple perforation plate; (9, 10), longitudinal tangential section showing rays and vessels, the arrow indicates a radial canal; (11), detail of longitudinal radial section showing heterocellular rays, the arrow indicates a prismatic crystal. Scale bars (1–5, 7–10) 100 μm; (6) 300 μm; (11) 50 μm.
Holotype
CIDPALBO-MEG 27, from the Ituzaingó Formation, Toma Vieja, Entre Ríos Province, Argentina.
Occurrence
Ituzaingó Formation (late Miocene? sensu Brunetto et al., Reference Brunetto, Noriega, Brandoni, Brandoni and Noriega2013), outcropping in Toma Vieja (CIDPALBO-MEG 88) and Arroyo El Espinillo (CIDPALBO-MEG 91), Entre Ríos Province, Argentina.
Description
The fossil specimens are in a very good state of preservation; CIDPALBO-MEG 88 has a diameter of 9 cm and is 28 cm long, and CIDPALBO-MEG 91 has a diameter of 5 cm and is 11.5 cm long. Growth rings are distinct, demarcated by a change in fiber diameter. Vessels are diffuse porous and circular in cross section, with tyloses and other deposits. Vessels are mainly solitary (53%), frequently in radial multiples of two (31%), sporadically in radial multiples of three (12%), rarely in radial multiples of more than three (2%), in tangential multiples (1%), and in clusters (1%). The mean vessel tangential diameter is 72 (35–130) μm in CIDPALBO-MEG 88 and 47 (26–69) μm in CIDPALBO-MEG 91, and the mean vessel radial diameter is 36 (12–71) μm in CIDPALBO-MEG 88 and 66 (10–120) μm in CIDPALBO-MEG 91. The mean vessel element length is 166 (100–245) μm. There are, on average, 30 (24–41) pores per mm2. The mean vessel wall thickness is 5 (2–6) μm. Vessel elements have straight to oblique end walls. Perforation plates are simple. Intervessel pits are bordered, alternately arranged, with an average diameter of 7 (4–9) μm. Vessel-ray pits have distinct borders and are like intervessel pits in size and shape throughout the ray cell. Fibers are polygonal to rounded in outline and arranged in radial rows. The mean fiber diameter is 13 (7–18) μm and the wall thickness is 4 (3–5) μm. Fibers have simple to minutely bordered pits. Axial parenchyma is paratracheal, vasicentric, scanty, confluent. Rays are heterocellular with one or more rows of square marginal cells; mainly uniseriate (52%) and biseriate (38%), and sporadically multiseriate (10%); 196 (40–440) μm high and 24 (10–70) μm wide, 11 (2–22) cells high and two (1–4) cells wide. There are 10 (6–14) rays per linear mm. Sheath cells are present. Prismatic crystals are present in rays. There are 1–2 radial canals in some multiseriate rays.
Materials
CIDPALBO-MEG 88, CIDPALBO-MIC 1127; CIDPALBO-MEG 91, CIDPALBO-MIC 1130.
Remarks
The new Ituzaingó material conforms to the specific diagnosis of Astroniumxylon bonplandianum.
Astroniumxylon parabalansae Franco and Brea, Reference Franco and Brea2008
Figure 5.3–5.5
- Reference Brea, Zucol and Patterer2010
Schinopsixylon heckii (Lutz); Brea, Zucol, and Patterer, p. 48, pl. V, figs. 1–8.
- Reference Franco2011
Incertae sedis; Franco, p. 220, fig. 7.42, 7.43.
Holotype
CIDPALBO-MEG 22, from the Paraná Formation (late Miocene), Toma Vieja, Entre Ríos Province, Argentina.
Occurrence
Ituzaingó Formation (late Miocene? sensu Brunetto et al., Reference Brunetto, Noriega, Brandoni, Brandoni and Noriega2013) outcropping at Curtiembre (CIDPALBO-MEG 78 and 81) and Toma Vieja (CIDPALBO-MEG 29), Entre Ríos Province, Argentina.
Description
The fossil specimens are in a very good state of preservation. CIDPALBO-MEG 29 is based on two fragments: the first one has a diameter of 7 cm and is 17.5 cm long, and the second one has a diameter of 3 cm and is 16 cm in long. CIDPALBO-MEG 81 has a diameter of 16 cm and is 19.5 cm long, and CIDPALBO-MEG 78 has a diameter of 8 cm and is 14 cm long. Growth rings are distinct, demarcated by marginal axial parenchyma. Vessels are diffuse porous. Vessels are circular in cross section with tyloses and other deposits. Vessels are mainly solitary (33%), frequently in radial multiples of two (32%), sporadically in radial multiples of three, four, five, and more elements (16%, 6%, 4%, and 5%, respectively), and in clusters (4%). The mean vessel tangential diameter is 86 (35–143) μm, and the mean vessel radial diameter is 64 (10–133) μm in CIDPALBO-MEG 78, 66 (10–120) μm in CIDPALBO-MEG 81 and 84 (63–103) μm CIDPALBO-MEG 29. The mean vessel element length is 171 (50–300) μm. There are, on average, 24 (18–36) pores per mm2 in CIDPALBO-MEG 78 and CIDPALBO-MEG 81, and 11 (6–13) pores per mm2 CIDPALBO-MEG 29. The mean vessel wall thickness is 6 (4–10) μm in CIDPALBO-MEG 78, 8 (4–18) μm in CIDPALBO-MEG 81, and 9 (6–14) μm in CIDPALBO-MEG 29. Vessel elements have straight to oblique end walls. Perforation plates are simple. Intervessel pits are circular, bordered, in alternate to subopposite arrangement, with an average diameter of 7 (6–9) μm in CIDPALBO-MEG 78 and CIDPALBO-MEG 81, and 4 (3–5) μm in CIDPALBO-MEG 29. Vessel-ray pits have much-reduced borders to apparently simple; pits are horizontal. Fibers are polygonal rounded in outline and arranged in radial rows. The mean fiber diameter is 12 (7–17) μm and the walls are 5 (3–6) μm. Axial parenchyma is paratracheal, vasicentric, lozenge-aliform, confluent, septate. Axial parenchyma bands are more than three cells wide and marginal. Rays are heterocellular with one or two rows of square marginal cells. Rays are mainly triseriate (32%), biseriate (28%) and uniseriate (25%), but tetraseriate (8%) or more (7%) occur. Rays are 189 (27–661) μm high and 26 (5–109) μm wide, nine (1–37) cells high and two (1–6) cells wide. There are 11 (7–14) rays per linear mm. Prismatic crystals are present in upright and/or square and procumbent ray cells. Some multiseriate rays have 1–2 radials canals.
Materials
CIDPALBO-MEG 29, CIDPALBO-MIC 679; CIDPALBO-MEG 78, CIDPALBO-MIC 1057; CIDPALBO-MEG 81, CIDPALBO-MIC 1058.
Remarks
The new Ituzaingó material conforms to the specific diagnosis of Astroniumxylon parabalansae.
Genus Schinopsixylon Lutz, Reference Lutz1979
Type species
Schinopsixylon herbstii Lutz, Reference Lutz1979 by original designation, from Ituzaingó Formation (late Miocene?), El Brete, Entre Ríos province, Argentina.
Schinopsixylon herbstii Lutz, Reference Lutz1979 emended
Figure 5.6–5.11
- Reference Lutz1979
Schinopsixylon heckii Lutz, p. 44, figs. 7–10.
- Reference Brea1999
Schinopsixylon heckii (Lutz); Brea, p. 64, figs. 2A–F, 3A–D.
- Reference Franco2011
Schinopsixylon heckii (Lutz); Franco, p. 201, fig. 7.36–7.38.
Holotype
PB-CTES 2925, from Ituzaingó Formation (late Miocene?), El Brete, Entre Ríos province, Argentina.
Emended specific diagnosis
Diffuse porous; vessels solitary and in radial multiples of 2–3 (4–5); vessel elements moderately numerous and medium sized; bordered and alternate intervessel pitting; abundant tyloses; perforation plate exclusively simple; rays mostly multiseriate, rarely uniseriate and biseriate; rays heterocellular, composed of procumbent and upright cells; rays multiseriate with canals; paratracheal axial parenchyma vasicentric and scanty.
Occurrence
Ituzaingó Formation (late Miocene? sensu Brunetto et al., Reference Brunetto, Noriega, Brandoni, Brandoni and Noriega2013) in Planta Potabilizadora, Paraná city, Entre Ríos Province, Argentina.
Description
The fossil specimen is in a good state of preservation and is based on two fragments: the first one has a diameter of 69 cm and is 141 cm long, and the second one has a diameter of 76 cm and is 222 cm long. Growth rings are distinct to indistinct. Vessels are diffuse porous. Vessels are circular in cross-section with tyloses and other deposits. Vessels are mainly solitary (67%), frequently in radial multiples of two (17%), sporadically in radial multiples of three and more elements (10% and 4%, respectively), and in clusters (2%). The mean vessel tangential diameter is 80 (46–111) μm and the mean vessel radial diameter is 96 (26–171) μm. The mean vessel element length is 176 (85–275) μm. There are, on average, 12 (8–15) pores per mm2. The mean vessel wall thickness is 11 (7–20) μm. Vessel elements have oblique end walls. Perforation plates are simple. Intervessel pits are circular to polygonal in shape, bordered, and alternately arranged, with an average diameter of 7 (5–7) μm. Vessel-ray pits have much-reduced borders to apparently simple; pits horizontal. Fibers are non-septate, polygonal in outline, and form the ground in radially oriented files. The mean fiber diameter is 12 (8–18) μm and the wall thickness is 5 (3–7) μm. Axial parenchyma is paratracheal, scanty, and vasicentric. Rays are heterocellular; body cells are procumbent and square and upright cells are mixed throughout the ray. The rays are mainly tetraseriate (28%) and triseriate (25%), biseriate (18%), uniseriate (14%), pentaseriate (12%), or more (3%). Rays are 247 (132–587) μm high and 32 (13–31) μm in wide, 12 (4–34) cells high and three (1–6) cells wide. There are eight (6–11) rays per linear mm. Prismatic crystals are present in upright and/or square ray cells. Some multiseriate rays have 1–2 radial canals.
Materials
CIDPALBO-MEG 70, CIDPALBO-MIC 1043.
Remarks
This specimen was coded according to IAWA convention for: presence of diffuse-porous wood (5p), simple perforation plates (13p), alternate intervessel pits (22p), tyloses (56p), non-septate fibers (66p), scanty paratracheal (78p) and vasicentric axial parenchyma (79p), rays 1–10 cells wide (97p and 98p), radial canals (130p), prismatic crystals in upright and/or square ray cells (136p and 137p); and for absence of ring-porous (3a) or semi-ring-porous (4a) wood, vessels in tangential bands (6a), vessels in diagonal and/or radial pattern (7a), vessels in dendritic pattern (8a). A search of the InsideWood database based on this coding returned four matches: Astronium graveolens Jacquin, Reference Jacquin1760; Astronium spp.; Schinopsis lorentzii (Grisebach) Engler, Reference Engler1881; and Schinopsis spp.
Based on the presence of diffuse porous, vessels solitary and in radial multiples of 2–5, abundant tylosis, perforation plate exclusively simple, bordered and alternate intervessel pitting, paratracheal axial parenchyma vasicentric and scanty, rays uniseriate to multiseriate, heterocellular, and rays multiseriate with canals, our material most closely resembles the extant genus Schinopsis and conforms to the circumscription of the fossil species Schinopsixylon herbstii (Lutz, Reference Lutz1979). We therefore emended the specific diagnosis to include the presence of solitary vessels and the phrase “rays heterocellular composed of procumbent and upright cells.”
Multivariate analyses
The cluster resulting from the analysis of 17 fossil specimens and four extant species shows three well-delimited groups: the first one is comprised by Metopium sp., PMET1 and PMET2; the second, by SCHK1, SCHT1, SCHK2, SCHK4 and Schinopsis balansae; and the third, Astronium balansae, Astronium urundeuva, all the specimens of Astroniumxylon and SCHK3 (Fig. 6.2). In this third group, it is possible to further distinguish three subgroups: the first one includes all the specimens of Astroniumxylon bonplandianum, the second has all the specimens of Astroniumxylon parabalansae and SCHK3, and the third includes Astronium balansae, Astronium urundeuva and Astroniumxylon portmannii.
The CA performed for the specimens indicates that the first four dimensions accounted for 63.781% of the total variation (Table 2). The first dimension accounts for 21.708% of the total variance (Table 2, Fig. 6). The characters that contributed most to the first dimension are: vessels in diagonal or dendritic pattern (3), porosity (2), shape (12) and arrangement (13) of intervessel pits, mean vessel element length (10), height of multiseriate rays and rays mostly 4–10 seriate (32) (Supplementary Material 3). Four characters—height of multiseriate rays (28), and rays mostly 4–10 seriate (32), shape of intervessel pits (12), and vessels in diagonal or dendritic pattern (3)—contributed most to the second dimension, which accounts for 17.672% of the total variance (Table 2 and Supplementary Material 3). Dimension three accounts for 12.801% of the total variance (Table 2); characters 13 (arrangement of intervessel pits), 10 (mean vessel element length), 29 (prismatic crystals in rays), and 30 (percentage of uniseriate rays) have the highest coefficients on this dimension (Supplementary Material 3). Dimension four accounted for 11.527% of the total variance; the arrangement of intervessel pits (13), percentage of uniseriate rays (30), apotracheal axial parenchyma (19), rays mostly 4–10 seriate (32), and vessels arrangement in diagonal or dendritic pattern (3) contributed most to this dimension (Supplementary Material 3). Therefore, the best definition of groups is represented by axis 1 versus 2 (together they explained 39.452% of variance), and three groups are clearly distinguished (Fig. 6.1).
Table 2. List of the eigenvalue and variance explained for each of the first four dimensions of the Correspondence Analysis (CA).

Discussion
Multivariate analyses
According to the cluster analysis, the group formed by PMET1, PMET2 (both specimens of Parametopioxylon crystalliferum n. gen. n. sp.), and Metopium sp. shows the lowest dissimilarity with values lower than 0.30. This is consistent with the fact that Parametopioxylon crystalliferum n. gen. n. sp. has more affinity with the descriptions made for Metopium sp. (see Terrazas, Reference Terrazas1994; InsideWood, 2004–onwards).
Another group is made up of different specimens of Schinopsixylon and Schinopsis balansae. In 1979, Lutz created the genus Schinopsixylon and erected two species, S. herbstii and S. heckii. According to the diagnosis, S. heckii differs from S. herbstii by the presence of mostly solitary pores (80% solitary pores and 20% in radial multiples) and only one radial canal per ray (Lutz, Reference Lutz1979). Based on our multivariate analysis, we determined that the characters 5 and 33 (vessels mostly solitary and number of radial canals per ray, respectively; Supplementary Material 1 and 3) lack enough taxonomic value to justify the separation of the two species in Schinopsixylon (Fig. 6) and correspond to intraspecific variations. Thus we proposed that S. heckii is synonymous with S. herbstii (Table 3; Fig. 6). These results are consistent with wood anatomy and multivariate analyses carried out by Gimenez et al. (Reference Giménez, Calatayu, Diaz Zirpolo, Figueroa and Gonzalez2015) in three species of extant Schinopsis (S. lorentzii, S. balansae, and S. marginata Engler, Reference Engler, De Candolle and De Candolle1883). These authors did not observe differences in the 29 anatomical characters studied and proposed that Schinopsis wood anatomy is substantially homogeneous with only some differences found in quantitative variables as well as density, diameter, and length of the vessels, all resulting from ecological adaptations caused by altitudinal gradient (Gimenez et al., Reference Giménez, Calatayu, Diaz Zirpolo, Figueroa and Gonzalez2015).
Table 3. Proposal of reassignment of the fossil specimens using multivariate analysis.

The characters enabling the distinction between Schinopsixylon and Astroniumxylon are the presence of rays mostly 4–10 seriate, exclusively paratracheal axial parenchyma, rays ≥6 cells wide common, and multiseriate rays commonly 301–400 μm in height (Supplementary Material 3). Therefore, as a result of our multivariate analysis, we transfer CIDPALBO-MEG 12 specimen (under the acronym SCHK3), previously assigned to Schinopsixylon heckii by Brea et al. (Reference Brea, Zucol and Patterer2010, see Table 1) to Astroniumxylon parabalansae on the basis of possessing apotracheal axial parenchyma and multiseriate rays ≤5 cells wide, and of not possessing prismatic crystals and mostly 4–10 seriate rays (Table 3, Fig. 6, Supplementary Material 3).
The CA delimits a group composed of Astronium balansae, A. urundeuva, and all the fossil woods with affinity to these. Two subgroups can be differentiated inside of that one. One subgroup consists of A. urundeuva and Astroniumxylon bonplandianum fossils; the other subgroup consist of A. balansae, Astroniumxylon parabalansae, and A. portmannii fossils (Fig. 6.1). In the cluster analysis, the fossil groups mentioned above are delimited, the extant species and A. portmannii are not related to the subgroups as in the CA (Fig. 6.2). Astroniumxylon parabalansae and Astroniumxylon portmannii can be differentiate because the first one has vessels in radial multiples of 4 or more elements and height of multiseriate rays are <300 μm.
Based on the multivariate analysis, ten variables are the most efficient in discriminating among species: porosity type (2), vessels arrangement (3), mean of vessel element length (10), shape (12) and arrangement (13) of intervessel pits, apotracheal axial parenchyma present (19), height of multiseriate rays (28), prismatic crystals in rays (29), percentage of uniseriate rays (30), and rays mostly 4–10 seriate (32). In the present paper, these characters are considered the most useful for delineating and circumscribing these fossil species. On the basis of our analyses, a diagnostic key for the species studied in this work is given:

Paleoclimatic, paleoecology and paleobiogeography inferences
The Anacardiaceae was an important component of the vegetation during the late Cenozoic in southernmost South America. This evidence is supported by the diverse and abundant Cenozoic fossil record of the family in this region.
Some peculiar features of the Anacardiaceae fossil woods, such as distinct growth rings, semi-ring-porous, radial canals, and scanty and paratracheal axial parenchyma, suggest a dry or seasonally dry warm climate. Others features present in some fossil woods from the Paraná Formation, such as wider vessels and low vessel frequency, suggest that some taxa were exceptionally adapted to humid conditions associated with riparian forests (see Brea and Franco, Reference Brea and Franco2013 and references herein).
Comparisons with the nearest living relatives of the Paraná fossil woods can also provide insight into paleoclimate and paleoecology. The genus Metopium includes evergreen to semi-deciduous, medium-size trees, ranging from West Indies to Florida, Mexico, and Central America (Terrazas, Reference Terrazas1994). This area corresponds to the Caribbean subregion sensu Morrone (Reference Morrone2001) and is known as Halffter's Mexican Transition Zone (MTZ). The MTZ is a complex area where the Neotropical and Nearctic biotas overlap (Halffter and Morrone, Reference Halffter and Morrone2017). The genus Astronium has endemic species with disjunct distribution throughout different areas in the Neotropical Dry Forests (Pennington et al., Reference Pennington, Prado and Pendry2000), many of which are characterized by a strongly seasonal climate. Astronium urundeuva is an important member of the Caatingaas, a seasonal dry forest in north-eastern Brazil (Prado and Gibbs, Reference Prado and Gibbs1993). Today, these genera do not occur where the fossils described here were collected. The finding of fossils with affinities to Metopium and Astronium affinity could be explained by a greater southward and eastward extent of Neotropical Dry Forests during the late Miocene than occurs today (Prado and Gibbs, Reference Prado and Gibbs1993; Pennington et al., Reference Pennington, Prado and Pendry2000; Reference Pennington, Lavin, Prado, Pendry, Pell and Butterworth2004; Prado, Reference Prado2000, DRYFLOR, 2016; Flanklin et al., Reference Franklin, Andrade, Daniels, Fairbairn, Gillespie, Gonzalez, Gonzalez, Imbert, Kapos, Kelly, Marcano-Vega, Meléndez-Ackerman, McLaren, McDonald, Ripplinger, Rojas-Sandoval, Ross, Ruiz, Steadman, Tanner, Terrill and Vennetier2018). These results are consistent with the fact that during the late Miocene, large areas of the continents experienced drying, enhanced seasonality, and a restructuring of terrestrial plant and animal communities. These global climate changes are seen throughout the subtropics, but have been attributed to regional tectonic forcing (Herbert et al., Reference Herbert, Lawrence, Tzanova, Peterson, Caballero-Gill and Kelly2016; Stevens Goddard and Carrapa, Reference Stevens Goddard and Carrapa2018).
Conclusions
A taxonomic revision and multivariate analyses were conducted on seventeen specimens of late Cenozoic Anacardiaceae fossil woods from northeastern Argentina and four extant species. Eight new silicified wood specimens assigned to four different species of the Anacardiaceae family were described: two specimens of Parametopioxylon crystalliferum n. gen. n. sp, three of Astroniumxylon bonplandianum, two of Astroniumxylon parabalansae, and one of Schinopsixylon heckii. Parametopioxylon crystalliferum n. gen. n. sp. has been erected based on exceptionally well-preserved fossil woods recovered from the Ituzaingó Formation.
A correspondence analysis of 22 anatomical characters of anacardiaceous woods was conducted. Taxonomically relevant characters, anatomical variability, and relationships among Anacardiaceae species were identified using correspondence and cluster analyses and allowed us to differentiate and revise the Anacardiaceae genera and species included in this paper. Schinopsixylon heckii Lutz, Reference Lutz1979 and Schinopsixylon herbstii Lutz, Reference Lutz1979 are not clearly segregated and can be explained by intraspecific or individual variability; we propose that S. heckii is synonymous of S. herbstii. Schinopsixylon could be distinguished from Astroniumxylon by the presence of mostly 4–10 seriate rays, commonly ≥6 cells wide, 301–400 μm in height, and axial parenchyma exclusively paratracheal. A taxonomic key is proposed in this paper to differentiate anacardiaceous fossil woods found in the late Cenozoic of northeastern Argentina.
Lastly, in accordance to previous studies, wood anatomy suggests that in the late Cenozoic, Anacardiaceae were linked to a dry or seasonally dry warm climate, with some trees and shrubs adapted to xeric conditions and seasonality and others exceptionally adapted to humid conditions associated with riparian forests.
Acknowledgments
The authors are grateful to A. Zucol, E. Passeggi, and C. Piña for their constructive and valuable comments, suggestions, and discussions during the preparation of this paper. This paper was financially supported by Agencia Nacional de Promoción Científica y Tecnológica PICT 2008-0176 (M. Brea and A.F. Zucol) and PICT 2014-1758 (M.J. Franco); FCyT-UADER PIDIN 2016 234-16 (M.J. Franco) and Consejo Nacional de Investigaciones Científicas y Tecnológicas PIP-CONICET 11220130100245CO (M. Brea and M.J. Franco). The authors would like to express their thanks to the anonymous reviewers and editors for their valuable help in providing critical and constructive comments.
Accessibility of supplemental data
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.x95×69pd3.
Appendix
List of all taxa used in the multivariate analyses
Genus Parametopioxylon n. gen.
Parametopioxylon crystalliferum n. gen. n. sp.
Holotype: CIDPALBO-MEG 41 (see Figs. 2–5 in this work)
Type stratigraphic horizon and age: Ituzaingó Formation, late Miocene?
Type fossil locality: Toma Vieja, Entre Ríos, Argentina.
Coding in this paper: PMET1.
Paratype: CIDPALBO-MEG 46, CIDPALBO-MIC 696.
Type stratigraphic horizon and age: Ituzaingó Formation, late Miocene?
Type fossil locality: Toma Vieja, Entre Ríos, Argentina.
Coding in this paper: PMET2.
Genus Astroniumxylon Brea, Aceñolaza, and Zucol, Reference Brea, Aceñolaza and Zucol2001
Type species: Astroniumxylon portmanii Brea, Aceñolaza, and Zucol, Reference Brea, Aceñolaza and Zucol2001
Holotype. MRVU 111 (see figs. 3.A–D and 4.A–D in Brea et al., Reference Brea, Aceñolaza and Zucol2001).
Type stratigraphic horizon and age: Paraná Formation, late Miocene.
Type fossil locality: Villa Urquiza, Entre Ríos, Argentina.
Coding in this paper: ASPO1.
Astroniumxylon parabalansae Franco and Brea, Reference Franco and Brea2008
Holotype: CIDPALBO-MEG 22 (see fig. 4.1–4.8 in Franco and Brea, Reference Franco and Brea2008).
Type stratigraphic horizon and age: Paraná Formation, late Miocene.
Type fossil locality: Toma Vieja, Entre Ríos, Argentina.
Coding in this paper: ASPA1.
Additional materials:
2009 Astroniumxylon parabalansae Franco and Brea in Franco, Ameghiniana 46: 587–604, 593, fig. 5.1–8 and 6.1–4 and in Franco, PhD Thesis: 1–343,188, fig. 7.33 and 7.35.
Material: CIDPALBO-MEG 26.
Coding in this paper: ASPA2.
2011 Astroniumxylon parabalansae Franco and Brea in Franco, PhD Thesis: 1–343,188, fig. 7.34 (CID-PALBO 78, CID-PALBO 81).
Material: CIDPALBO-MEG 78.
Coding in this paper: ASPA3.
Material: CIDPALBO-MEG 81.
Coding in this paper: ASPA4.
This paper. Astroniumxylon parabalansae Franco and Brea in this work.
Material: CIDPALBO-MEG 29.
Coding in this paper: ASPA6.
Synonyms:
2010 Schinopsixylon heckii Lutz in Brea et al., Reference Brea, Zucol and Patterer2010, Review of Palaeobotany and Palynology 163: 35–51, 48, pl. V 1–8.
Material: CIDPALBO-MEG 12.
Coding in this paper: SCHK3.
2011. Insertae sedis 1 Franco, PhD Thesis: 1–343, 220, fig. 7.42–7.43 (CID-PALBO 42).
Coding in this paper: ASPA5
Astroniumxylon bonplandianum Franco, Reference Franco2009
Holotype: CIDPALBO-MEG 27 (see fig. 3.1–3.6 and 4.1–4.7 in Franco, Reference Franco2009).
Type stratigraphic horizon and age: Ituzaingó Formation, late Miocene?
Type fossil locality: Toma Vieja, Entre Ríos, Argentina.
Coding in this paper: ASBO1.
Additional material:
2011 Astroniumxylon bonplandianum Franco in Franco, PhD Thesis: 1–343,184.
Material: CIDPALBO-MEG 88.
Coding in this paper: ASBO2.
2011 Astroniumxylon bonplandianum Franco in Franco, PhD Thesis: 1–343,184.
Material: CIDPALBO-MEG 91.
Coding in this paper: ASBO3.
Genus Schinopsixylon Lutz, Reference Lutz1979.
Type species: Schinopsixylon herbstii, Lutz Reference Lutz1979.
Holotype: PB-CTES 2925 (see figs. 1–6 in Lutz, Reference Lutz1979).
Type stratigraphic and age: Ituzaingó Formation, late Miocene?
Type fossil locality: El Brete, Entre Ríos, Argentina.
Coding in this paper: SCHT1.
Synonyms:
1979 Schinopsixylon heckii Lutz, Reference Lutz1979, Facena 3: 39–63, 44, fig. 7–10.
Material: PB-CTES 4826.
Coding in this paper: SCHK1.
1999 Schinopsixylon heckii Lutz in Brea, Reference Brea1999, Ameghiniana 36: 63–69, 64, fig. 2.A–F, 3.A–D.
Material: LPPB 1281.
Coding in this paper: SCHK2.
2011 Schinopsixylon heckii Lutz in Franco, PhD Thesis: 1–343, 201, fig.7.36–7.38.
Material: MAS PALEOBOT 266 and CIPALBO-MEG 70 (isotype).
Coding in this paper: SCHK4.












