About This Column
Aaron Kesselheim serves as the editor for Health Policy Portal. Dr. Kesselheim is the JLME editor-in-chief and director of the Program On Regulation, Therapeutics, And Law at Brigham and Women's Hospital/Harvard Medical School. This column features timely analyses and perspectives on issues at the intersection of medicine, law, and health policy that are directly relevant to patient care. If you would like to submit to this section of JLME, please contact Dr. Kesselheim at akesselheim@bwh.harvard.edu.
For patients living with a life-threatening disease and few or no meaningful treatment options, the COVID-19 pandemic response is something to behold. Unapproved products have been granted Emergency Use Authorization,1 clinical trials have been launched at a remarkable pace,2 and investigational products have been made available for widespread treatment use.3 Frustrated patients might reasonably wonder why we have not responded to amyotrophic lateral sclerosis (ALS), metastatic cancer, or any other life-threatening condition with the same “pull-out-the-stops” mentality.Reference Green4 Although there are many differences — legally, politically, and scientifically — that set a novel infectious disease outbreak apart, the best answer is that even when facing dire consequences, it is critical not to inappropriately abridge the process of drug development.5 Instead, we must perpetually balance speed and safety, access and data collection, short- and long-term goals, whether for novel conditions such as COVID-19 or for threats to human health that have been with us much longer. Failure to achieve this balance can lead to unsafe and ineffective products at one extreme and unacceptable delay in meeting patients' needs at the other.Reference Sharfstein6
The Food and Drug Administration (FDA) has tools at its disposal to facilitate this balance, but recent efforts such as the Right to Try movement have sought to dangerously tip the scales.Reference Joffe, Lynch, Lynch, Zettler and Sarpatwari7 Another piece of recently proposed legislation, “The Promising Pathway Act,” takes a further step in the wrong direction. The bill intends to speed patient access to potentially life-saving medicines — an undoubtedly worthy goal — but would do so via a new pathway that could inhibit the ability to understand whether those products are truly safe and effective. Instead of new approaches to marketing approval, policymakers would do better by improving FDA's existing Accelerated Approval pathway and, more importantly, acknowledging the shortcomings of approaches that grant marketing approval based on an initially limited evidence base intended to be buttressed by postapproval data collection. Whether for pandemic infections or for other devastating conditions, we can accommodate the interests of both current and future patients by offering pre-approval access to promising therapies through FDA's Expanded Access pathway while conducting the essential, rigorous studies that become much more difficult once a product is approved for marketing. Policymakers should embrace Expanded Access and focus on eliminating unintended barriers facing patients, doctors, and companies seeking to use it, rather than pursuing yet another modification to marketing approval.
The Promising Pathway Act
On December 19, 2019, the predecessor to the Promising Pathway Act — a legislative proposal called the Conditional Approval Act — was introduced in both the House (H.R. 5497) and Senate (S. 3133). That bill proposed to allow sponsors of new drugs intended for treatment, prevention, or diagnosis of serious, life-threatening, or chronic diseases or conditions to seek “provisional and time-limited approval” for marketing if the drug's expected benefits outweigh potential risks to patients; the sponsor will likely be able to provide “comprehensive clinical data” after approval; confirmatory clinical trials would be difficult or costly to conduct; and there is a dearth of meaningful alternatives. If the sponsor did not secure marketing approval based on a “full demonstration of safety and effectiveness” within 5 years, Conditional Approval would expire. The bill's sponsors explained that it was intended to help small companies get their products to market without the expense of Phase III trials, ostensibly so that they could “give patients access to innovative treatments and compete with large, monopolistic pharmaceuticals to lower consumer cost,” while using the resultant profits to fund studies that may have been cost-prohibitive for a company without any marketed products.8
Even if Accelerated Approval could be improved, it entails a fundamental tradeoff between the potential benefits of early patient access and the difficulty of collecting rigorous evidence postapproval. Accelerated Approval can be a useful tool nonetheless, but when there are other ways to secure patient access, they should be preferred.
The sponsors sought comments on the Conditional Approval Act and received feedback and proposed revisions from hundreds of stakeholders and experts, leading to a new proposal dubbed the Promising Pathway Act, formally introduced in the Senate (S. 3872) on June 4, 2020.9 Touted as an approach to avoiding a “bureaucratic journey through red tape and regulations,”10 but without mention of lowering drug costs, this proposal is even more expansive than its predecessor. It would apply to drugs for the treatment, prevention, or diagnosis of a “serious or life-threatening disease or condition for which there is a reasonable likelihood that premature death will occur without early medical intervention,” as well as “a disease or condition associated with morbidity that has a substantial impact on day-to-day functioning.” As a sign of how things have changed since December 2019, newly added to this proposal are diseases or conditions posing “a threat of epidemic or pandemic.”
The crux of the proposal is a new provisional approval pathway based on “substantial evidence of safety” and “relevant early evidence based on adequate and well-controlled investigations, including early-stage clinical investigations, to establish that the drug provides a positive therapeutic outcome.” Where there is already a currently marketed on-label therapy for the condition the new drug is intended to treat, provisional approval would require outcomes “consistent with or greater than” those offered by available drugs, with equal or fewer side effects. In terms of what might count as “early evidence,” provisional approval could be based on “real world evidence” and “scientifically-substantiated surrogates,” meaning surrogate endpoints that have not been validated by FDA but that are thought to potentially predict clinical benefit based on “epidemiologic, therapeutic, pathophysiologic, or other evidence; or an effect on a clinical endpoint other than survival or irreversible morbidity of interest.” To promote speed, the proposal includes provisions for rolling review of application components prior to completion of a full dossier and would require evaluation of a completed application within 90 days (or 3 weeks for epidemic or pandemic drugs).
Eligible drugs meeting these evidentiary standards could receive provisional approval in 2-year increments for a maximum total of 6 years. In order to access a drug under provisional approval, patients would be required to provide written informed consent acknowledging acceptance of the risks of taking a drug that has not yet received full approval. They would also be required to participate in an “observational registry” and consent to collection and submission to the registry of data related to the patient's use of the drug. The bill does not specify the precise data that would be required for inclusion in the registry, but mentions “patient treatments and uses, length of use, side effects encountered, relevant bio-markers or scientifically substantiated surrogates, scan results, cause of death and how long the patient lived, and adverse drug effects.”
Registries would be used in a number of ways, with data available to both patients and researchers. FDA would be required to conduct annual reviews to assess whether a drug's side effect profile fails to “support the benefit provided, or the data shows the benefit is less than the benefits offered through other, fully-approved drugs.” FDA could levy financial penalties and withdraw provisional approval if fewer than 90% of patients using the product are participating in the relevant observational registry. Provisional approval could also be revoked due to safety concerns.
Since provisional approval is intended to be temporary and would come with an expiration date, the proposal also discusses steps toward “full approval” under FDA's traditional marketing approval requirements.11 Provisionally approved drugs that establish a 15% improvement in “an important endpoint,” including unvalidated surrogates, “compared to a standard drug” would automatically be granted full approval. The intended requirements for full approval of other provisionally approved drugs, including when there is no other approved product for a given indication, are less clear. There appear to be no specific post-approval requirements other than the patient registries described above; in particular, the bill makes no mention of required post-approval trials. The proposal would require FDA to allow use of real world evidence, i.e., from sources other than randomized controlled trials, to support applications for full approval, but it is unclear whether data from the observational registries is intended to suffice.
Table 1. Improving Existing FDA Pathways for Early Patient Access to Drugs
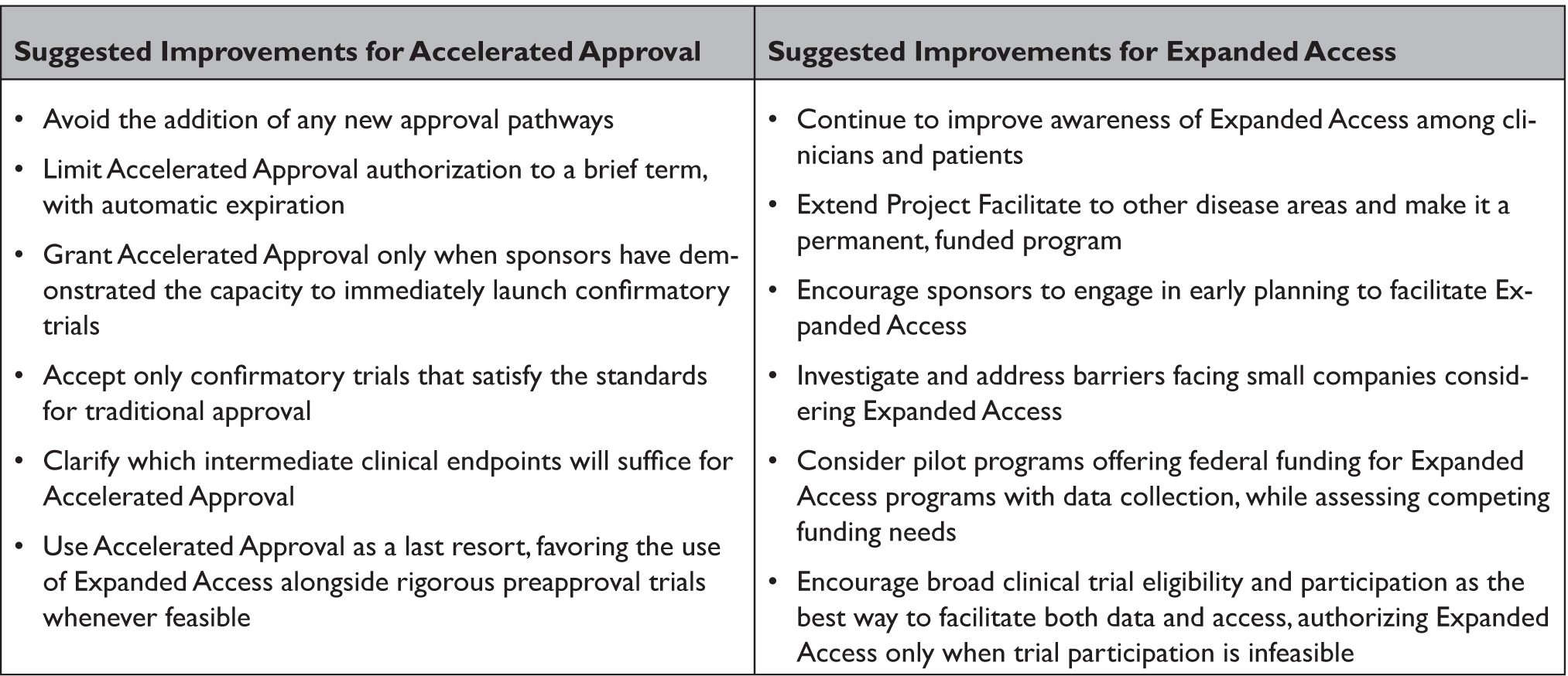
In order to promote patient access to provisionally approved products, the proposal would prohibit health insurers, including federal health care programs, from denying coverage on the basis that a provisionally approved drug is “experimental,” an issue that has plagued some Accelerated Approval products. Instead, they would be required to treat provisionally approved drugs in the same manner as drugs fully approved under other pathways, although parity in this regard does not necessarily require coverage. Other components of the proposal describe annual reports from FDA on use of this pathway, liability limitation for sponsors and manufacturers, and specific policies for vaccines intended to address epidemic disease.
It is unclear what final form this proposal will take and what level of Congressional support it will ultimately muster. However, approaches that push for early access to new drugs have been a key tenet of advocacy for some patient organizations, particularly in areas of severe unmet need, like ALS, rendering this legislative activity an important opportunity to explore the challenges generated by facially compassionate and seemingly common-sense policy proposals.
Improving Accelerated Approval
Although the Promising Pathway Act is a new bill, the idea of conditional or provisional approval is not. For a new drug expected to offer meaningful therapeutic benefit over existing treatments for a serious or life-threatening disease or condition, FDA's existing Accelerated Approval pathway allows marketing approval based on unvalidated surrogate endpoints reasonably likely to predict clinical benefit or intermediate clinical endpoints likely to predict an effect on irreversible morbidity or mortality,12 similar to the “scientifically-substantiated surrogates” contemplated for provisional approval.13 However, sponsors of drugs granted Accelerated Approval are explicitly expected to conduct postapproval studies to verify clinical benefit “with due diligence.” If they fail to do so, or if a required study fails to verify the predicted benefit or other evidence demonstrates a lack of safety or efficacy, FDA may withdraw approval.14 In contrast, the Promising Pathway Act appears not to require post-approval trials or ultimate confirmation of benefit based on validated endpoints, potentially relying on observational registry data to support full approval.
Experience with Accelerated Approval over three decades gives reason for concern about provisional approval approaches more broadly,Reference Miller, Joffe, Herder, DiMagno, Glickman and Emanuel15 with similar challenges exhibited abroad.Reference Banzi and Hoekman16 Many confirmatory trials for drugs marketed on the basis of Accelerated Approval are ongoing or delayed, often due to recruitment challenges.Reference Beaver, Naci, Smalley, Kesselheim and Herper17 When they are completed, FDA has been willing to accept weak evidence, sometimes relying on the same surrogate endpoint used in the preapproval trial,Reference Gyawali, Hey and Kesselheim18 and to allow products to remain on the market even after confirmatory trials fail to verify clinical benefit.Reference Rubin19 Trials conducted after Accelerated Approval also often lack important design characteristics that contribute to scientific confidence, such as blinding, randomization, and concurrent comparator groups.20 As a result of these shortcomings, patients may be exposed for too long to drugs that may not be safe and effective, risking disease progression and additional harm, wasting time and money, distracting from trials of products that may be better, and diverting from pursuit of other alternatives, potentially including palliation.Reference Vitry, Nguyen, Entwistle and Roughead21 Each of these concerns would be replicated by the approach contemplated under the Promising Pathway Act and exacerbated if drugs were permitted to remain on the market after the maximum provisional approval period on the basis of unvalidated surrogates or observational registry data alone.
Whether for COVID-19, ALS, or any other life-threatening disease, there is an understandable desire to remove traditional barriers to product development in hopes of saving lives. FDA has a number of pathways available to do just that, but it is critical not to lose sight of foundational goals of preapproval safety and efficacy review. Ultimately, what all patients, clinicians, and payers need is strong evidence that a drug provides sufficient benefit to justify its possible risks. There may be reason to accept short-term uncertainty to facilitate patient access when time is of the essence, but achieving that access via premature marketing approval impedes, and in some situations could outright prevent, the accumulation of high-quality confirmatory evidence postapproval. Instead, it is preferable to utilize and improve existing preapproval access options that prioritize the completion of rigorous clinical trials before marketing.
Rather than creating new approval options, it would be preferable to strengthen the existing Accelerated Approval pathway to encourage meaningful, timely confirmatory trials. First, the Promising Pathway Act is right that provisional approval should not be permitted to extend indefinitely, since that risks insufficient incentive for sponsors to push toward confirmation of genuine benefit. Accelerated Approval therefore should only be granted for a brief, renewable, limited term, followed by automatic expiration absent satisfactory confirmation of safety and efficacy during that time. To provide some indication of feasibility and minimize use of this pathway for drugs unlikely to be able to provide adequate confirmatory evidence, Accelerated Approval should only be granted if confirmatory trials are ready to enroll participants or already underway.23
Second, FDA should only accept confirmatory trials that meet the current standard for traditional marketing approval, i.e., those utilizing clinical outcomes or established surrogate endpoints specifically validated for the drug's indication,Reference Wallach, Ross, Naci, Ciani, Gyawali, Hey and Kesselheim24 with care not to allow slippage in what counts as validated.Reference Piller25 That said, there is also an important need to identify acceptable new endpoints that are meaningful to patients and caregivers, which may be more subjective than traditional biomedical endpoints. Overall, efforts to weaken or eliminate requirements for rigorous postapproval trials should be resisted.
Challenges of Collecting Rigorous Data Postapproval
Even if Accelerated Approval could be improved, it essential to recognize that it – and any pathway that permits marketing approval before safety and effectiveness has been well-demonstrated – entails a fundamental tradeoff between the potential benefits of early patient access and the difficulty of collecting rigorous evidence post-approval. Accelerated Approval can be a useful tool nonetheless, but when there are other ways to secure patient access, they should be preferred.
When we wait until after a product is approved to gather evidence as to whether it is truly safe and effective, we may be accepting extended or even perpetual uncertainty. Take a new drug granted Accelerated Approval for a serious or life-threatening disease or condition currently without alternative treatments. Without better options, many patients will understandably flock to it, even if there is only weak evidence of effectiveness. (Note that this was precisely the concern with regard to off-label hydroxychloroquine for treatment of COVID-19, although not an Accelerated Approval example.Reference Piller25) But this means that randomized controlled postapproval trials will likely be at best extremely challenging, and at worst infeasible, because patients — and perhaps their physicians — are reluctant or unwilling to forgo the new drug, making it difficult to gather strong confirmatory evidence.Reference Mello and Brennan26 Moreover, concerns about feasibility will be compounded for any subsequent investigational drug for the same indication. This is because patients will likely enroll in a trial evaluating the next agent only if it is tested against the available drug, not placebo. Yet comparing a new product against one never compellingly demonstrated to be safe and effective cannot result in a compelling demonstration of the new product's safety and efficacy, even if it is found to be superior to the earlier comparator. And so on.27 Although real world evidence may help, experts most often consider it a complement to, rather than substitute for, randomized controlled trials (RCTs).Reference Mahendraratnam28
Studies lacking traditional features of rigorous design can sometimes produce compelling evidence of safety and efficacy, but this demands strong understanding of the disease's biology and the drug's mechanism of action, large effect size, and well-understood, consistent, and typically poor outcomes for patients on current therapy or supportive care.Reference Miller, Joffe, Druker and Elliott29 More often, conditions present with substantial variability within and across patients, and treatment effects are relatively modest. In these circumstances, RCTs — not only observational registries — are needed to provide patients and clinicians with critical information to guide treatment decisions.Reference Darrow, Avorn, Kesselheim and Zhang30 They are also important to providing payers with information to guide decisions about how to spend limited health care dollars.31 Without strong evidence, payers may be appropriately reluctant to reimburse for approved drugs, regardless of their approval pathway.Reference Thomas32 Perhaps recognizing this, the Promising Pathway Act does not purport to require reimbursement, but rather calls for parity in reimbursement decisions for provisionally and conventionally approved products.
The reality is that one function of requiring FDA approval prior to marketing is to restrict whether and how patients can access investigational drugs so that rigorous clinical testing becomes possible.Reference Kapczynski33 The options available to current patients are limited in large part for the benefit of future patients, while current patients benefit from contributions to clinical advancement made by patients who came before them. By contrast, allowing earlier marketing approval based on reduced evidentiary standards risks sacrificing the longer-term interests of all patients in safe and effective products in favor of the shorter-term interests of seriously ill patients who are willing to forgo strong evidence because they lack meaningful alternatives. Yet, this tradeoff can be avoided. It is possible to have our data and access, too.
Removing Barriers to Expanded Access
Rather than promoting weaker approval standards, provisional or otherwise, in order to accommodate patients with unmet therapeutic needs, it may be possible to make better use of FDA's Expanded Access pathway. Expanded Access allows patients with serious or life-threatening diseases or conditions who have exhausted approved treatment options to access promising investigational drugs before approval and outside a trial, if the sponsor agrees to provide them, FDA signs off, and an Institutional Review Board approves. Importantly, it must also be the case that this access will not interfere with clinical trials or otherwise derail clinical development. Expanded Access can be authorized for single patients, intermediate-size populations, or widespread populations, each with somewhat different regulatory requirements.34
FDA has taken several steps to streamline this pathway, but the total number of Expanded Access requests (some of which cover multiple patients) remains relatively low — averaging about 1,800 applications annually, nearly all of which are ultimately authorized by FDA.35 This number will reflect a significant jump for 2020 based on a large number of requests for investigational products for COVID-19,Reference O'Day36 and may remain elevated due to increasing attention to this option. Yet, it is not clear what the optimal level of Expanded Access ought to be, given that the drugs in question have not yet been approved as safe and effective on any standard. What is clear, however, is that with appropriate safeguards for both desperate patients and data generation, the risks and benefits of trying an unapproved drug can sometimes be reasonable for a patient or group of patients, especially as a product proceeds through clinical development and promising evidence begins to accumulate. Thus, it is also reasonable to remove unintended barriers to Expanded Access.
FDA has already begun to address barriers related to physician awareness and the burdens associated with making requests, for example through its pilot oncology program, “Project Facilitate.”37 Launched in 2019, this program offers “concierge” services to help physicians navigate Expanded Access, including liaising with companies and assistance with submission requirements. Broadening Project Facilitate to other disease areas and creating a permanent staff and budget for it could promote more equitable access and better awareness of this pathway.
Another critical barrier is that even sponsors willing to provide Expanded Access may find that it is logistically impossible to do so without early planning that begins well before the first patient request. To address this challenge, FDA could require sponsors to submit product-specific Expanded Access plans prior to moving into Phase II trials. These plans would be more detailed — covering scope of access, eligibility, and timing — than the general policies regarding evaluating and responding to requests for Expanded Access that companies developing investigational drugs are required to make public under the 21st Century Cures Act.38 Although sponsors would remain free to refuse Expanded Access, encouraging early, product-specific consideration in coordination with regulators may prevent such refusals from arising on the basis of inertia or lack of attention. It would also normalize and routinize discussions about Expanded Access throughout a product's development.
Small companies may also face unique impediments to Expanded Access. First, they may be especially concerned that negative outcomes reported in Expanded Access patients will influence investors, even though FDA has emphasized its ability and willingness to appropriately contextualize this information without automatically attributing harm to the investigational product, thereby avoiding inappropriate negative impact on its clinical development and ultimate approval.39 Thus, FDA should continue to repeat and spread this message.
Second, small companies may face considerable financial constraints that make it difficult to cover the costs of manufacturing and providing product to meet Expanded Access requests, as well as the personnel needed to manage them.40 Importantly, even though patients can be charged for direct costs of drugs provided via Expanded Access, doing so raises justice concerns and, in any case, may not be timed in a way that would produce the cash flow needed to allow small companies to take critical preparatory steps.Reference Folkers, Bateman-House and Robertson41 FDA should investigate these challenges in collaboration with relevant stakeholders and consider what solutions are feasible.
One approach currently being considered in Congress entails federal government subsidies for Expanded Access. A recent legislative proposal called the Accelerating Access to Critical Therapies for ALS Act would make $75 million available annually for 2 years, and twice that available over the next 2 years, as part of a pilot to provide grants to Expanded Access programs for rapidly progressing neurodegenerative disease.42 Although likely to be effective in improving access for patients, this approach also raises justice concerns about the use of government funds for unproven therapies (especially if focused on only a single clinical area) while there are many unmet healthcare needs related to proven medical interventions. There are also important questions about how best to allocate funds as between further research and Expanded Access. To the extent that Expanded Access is combined with data collection, however, some ethical concerns about the use of federal funds potentially can be partially mitigated.
Finally, although Expanded Access is preferable to Accelerated Approval or other pathways to early marketing approval when uncertainty remains about safety and efficacy, enrolling more patients in rigorous preapproval trials is preferable to both.43 Policy-makers should therefore continue recent efforts encouraging sponsors to broaden trial eligibility criteria, allowing more patients access while collecting data that can meaningfully inform the likely range of actual clinical use.44
Conclusion
Whether for COVID-19, ALS, or any other life-threatening disease, there is an understandable desire to remove traditional barriers to product development in hopes of saving lives. FDA has a number of pathways available to do just that, but it is critical not to lose sight of foundational goals of preapproval safety and efficacy review. Ultimately, what all patients, clinicians, and payers need is strong evidence that a drug provides sufficient benefit to justify its possible risks. There may be reason to accept short-term uncertainty to facilitate patient access when time is of the essence, but achieving that access via premature marketing approval impedes, and in some situations could outright prevent, the accumulation of high-quality confirmatory evidence postapproval. Instead, it is preferable to utilize and improve existing preapproval access options that prioritize the completion of rigorous clinical trials before marketing.
Acknowledgments
This article is based on a letter sent to Congressmen Bruce Westerman, Mike Gallagher, and Tim Burchett, and Senators Mike Braun and Lisa Murkowski on February 3, 2020, in response to Senator Braun's request for comments on the proposed Conditional Approval Act. The letter, available at https://bit.ly/CAALetter, was led by Holly Fernandez Lynch and signed by Steven Joffe, Patricia J. Zettler, Arthur L. Caplan, Jonathan J. Darrow, Alison Bateman-House, Ameet Sarpatwari, Jeremy Snyder, Huseyin Naci, Christopher T. Robertson, Jinsy A. Andrews, and Aaron S. Kesselheim, each in their individual capacity and not representing their respective institutions. Although related, the contents of this article should be attributed only to the authors and do not necessarily reflect the views of the letter's signatories. Thank you to Steven Joffe for extensive discussion of these issues.



