Introduction
Residual cholesteatoma most commonly occurs in hidden recesses such as the sinus tympani.Reference El-Meselaty, Badr-El-Dine, Mandour, Mourad and Darweesh1 Rigid endoscopes, which provide illumination and a wide-angled field of view, give an enhanced view into middle-ear recesses, and have been used for many years to improve the detection of cholesteatoma remnants.Reference El-Meselaty, Badr-El-Dine, Mandour, Mourad and Darweesh1–Reference Presutti, Marchioni, Mattioli, Villari and Alicandri-Ciufelli3 Retro-tympanic cholesteatoma remnants have been found with endoscopy in around one-third of cases after attempted clearance using an operating microscope.Reference El-Meselaty, Badr-El-Dine, Mandour, Mourad and Darweesh1,Reference Presutti, Marchioni, Mattioli, Villari and Alicandri-Ciufelli3–Reference Bennett, Wanna, Francis, Murfee, O'Connell and Haynes5 Endoscopes have been used with growing enthusiasm, especially over the last decade, to facilitate dissection from this challenging location.Reference Presutti, Marchioni, Mattioli, Villari and Alicandri-Ciufelli3,Reference Tarabichi6–Reference James11
Cholesteatoma matrix can be trapped beyond the reach of mechanical dissection in a deep sinus tympani or under an intact ossicular chain. In such circumstances, a potassium titanyl phosphate (KTP) laser can be used with endoscopic guidance to effectively remove cholesteatoma, without the need for more destructive surgery that would be otherwise required to open up such hidden recesses for access.Reference le Nobel and James12
This paper describes techniques for the safe and effective use of the KTP laser during transcanal totally endoscopic cholesteatoma surgery, and describes our experience and outcomes using this approach.
Materials and methods
A prospective cohort study was performed on all children who underwent totally endoscopic cholesteatoma surgery with the use of the KTP laser for cholesteatoma, over an eight-year period, at a tertiary referral children's hospital. Research Ethics Board approval for this study was obtained from the Hospital for Sick Children, Toronto (Research Ethics Board numbers: 1000012951 and 1000033566).
The main outcome measures were: the proportion of cases in which the KTP laser was required, the reasons for KTP laser use, the residual cholesteatoma rate and the strategies for facilitating KTP laser use. The primary outcome measure was the presence of residual cholesteatoma. Residual disease most commonly takes a pearl-like cystic form, though can be observed as an open or en plaque sheet. It is not in continuity with the squamous epithelial surface of the tympanic membrane or external auditory canal. Cases of recurrent cholesteatoma arising from a new retraction of the tympanic membrane into the tympanomastoid system were not included in this analysis.
The inclusion criteria were: patients aged less than 18 years, new cases of cholesteatoma (stage = S1), surgery completed with totally endoscopic cholesteatoma surgery (approach = A1), and adequate follow up (more than 1 year from surgery and not awaiting planned second-stage surgery).Reference Yung, James, Merkus, Philips, Black and Tono13
Surgical technique
Totally endoscopic cholesteatoma surgery was only used for cholesteatoma predominantly localised in the middle ear and attic, but not extending beyond the lateral semicircular canal. The endoscopes used were 0°, 30° or 45°, 3 mm in diameter and 14 cm in length. Second-stage surgery was planned after an interval of 12 months for cases in which cholesteatoma extended into the epitympanum, or if there were concerns about potentially incomplete matrix removal (e.g. piecemeal dissection, bleeding granulation tissue), or when the limits of disease were uncertain (e.g. in open or en plaque disease).
A KTP laser was used for the purposes of: (1) ablating granulation tissue without bleeding; (2) removing cholesteatoma from ossicles, especially the stapes, to avoid the risk of mechanical trauma causing cochlear injury;Reference James and Takahashi14,Reference Hamilton15 and (3) ablating unseen cholesteatoma remnants, to reduce the risk of residual disease.Reference Hamilton16 The KTP laser was occasionally used to shrink atelectatic segments of the pars tensa and to remove delicate fragments of bone (e.g. in congenital ossicular fixation).Reference Badr-El-Dine, James, Panetti, Marchioni, Presutti and Nogueira10,Reference Brawner, Saunders and Berryhill17
One significant advantage of the KTP laser is that it is carried by a semi-flexible glass fibre, which is easily manipulated within the ear and is relatively inexpensive. Importantly, the fibre-optic carrier is narrow enough to be used permeatally alongside an endoscope. The carrier is a metal sheath that holds the optical fibre, and the tip can be bent gently to allow access to the depth of recesses that are revealed by angled endoscopes. It is important to ensure that the tip does not become broken or coated with carbon.Reference Tracy, Kobler, Van Stan and Burns18
In order to maintain a clear view of the operative field when using a KTP laser with an endoscope, a 532 nm filter (AMS Solutions for Life, San Jose, California, USA) was placed between the camera and endoscope to eliminate glare. The camera was white-balanced after placement of the filter. Protective eye glasses were worn by operating theatre personnel. Our experience found it to be advantageous for the surgeon to look over the top of the protective glasses, to have a clear view of the surgical field on the monitor while still shielding the eyes from the laser. Safety was enhanced by keeping the laser on standby when the tip of the fibre-optic carrier was not in the ear.
Tissue vaporisation during laser use in totally endoscopic cholesteatoma surgery generated a smoke plume that quickly obscured the endoscopic view, and could not easily be cleared by the surgeon while holding the endoscope in one hand and the laser carrier in the other. We found several solutions to this: (1) an assistant can hold a suction cannula in the ear canal, but it can sometimes be difficult safely to attain appropriate proximity to middle-ear space without bumping into the endoscope or fibre; (2) a non-flammable suction cannula can be attached to the endoscope (Figure 1); or (3) most effectively, the suction cannula can be attached to the laser carrier (Figure 2). An alternative method of using a suction cannula as a carrier for the laser fibre has been described elsewhere.Reference Clark and Commins19

Fig. 1. Malleable cannula attached to endoscope for suction of laser smoke plume.

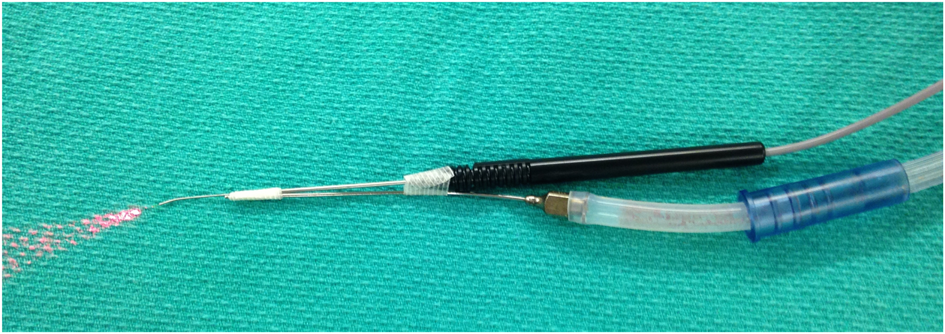
Fig. 2. Suction cannula attached to laser fibre carrier.
The KTP laser was used at power settings between around 0.3 W (e.g. on the stapes superstructure) and 1 W (e.g. with a defocused beam to ablate potentially unseen remnants). Contact of the laser fibre with tissue tended to weld carbonised tissue to the tip of the fibre. This was used to safely pull matrix off the stapes. Prolonged laser activity at one spot was avoided, to minimise the risk of collateral thermal spread to adjacent tissues. The laser was not used over the facial nerve.Reference Eskander, Holler and Papsin20
Pre-operatively, high-resolution computed tomography scans were studied to understand the patient's retro-tympanic microanatomy, and facial nerve monitoring was used to help safeguard the nerve.
Results
A total of 148 consecutive patients underwent totally endoscopic cholesteatoma surgery for a new case of cholesteatoma confined to the middle ear and attic. However, 36 of these patients were excluded from the study as they had less than one year of follow up, and 8 were excluded as they were still awaiting planned second-stage surgery or magnetic resonance imaging (MRI) screening for residual cholesteatoma. Twenty-one additional patients were excluded, having been categorised as ‘pre-cholesteatoma’ cases (including cases of severe progressive tympanic retraction containing cleanable keratin or granulation), of which none developed residual cholesteatoma.
Of the remaining 83 patients (mean age of 10.5 years, range = 1.8–18 years), acquired cholesteatoma originated in: the pars tensa in 42 cases, the pars flaccida in 15 cases and both areas in 4 cases. Fifteen cases were congenital and a further seven cases were of uncertain, possibly congenital origin. Second-stage surgery was conducted in 46 patients (55 per cent) and non-echo-planar diffusion-weighted MRI screening was carried out in 4 patients (mean of 3.2 years post-operation); the remaining 33 patients (40 per cent) were monitored with observation in clinic (mean follow up of 3.0 years (range, 1–7.5 years)).
A KTP laser was used in 70 of the 83 cases (84 per cent) that met the inclusion criteria. The laser was not used in 13 ‘clean’ cases in which the disease was removed more easily.
Residual disease was detected in 5 out of 83 cases (6 per cent), of which the KTP laser had been used in 4 cases (5 per cent). Hence, residual disease was detected in 4 of the 70 cases (6 per cent) treated using a KTP laser.
Details of the locationReference Yung, Tono, Olszewska, Yamamoto, Sudhoff and Sakagami21 of initial and residual disease are summarised in Table 1. Several of the residual pearls were found in close proximity to the facial nerve, where the KTP laser was not applied. One residual pearl was found in a hidden site around the anterior surface of the head of the malleus, an anticipated consequence of attempting to preserve an intact ossicular chain. Of note, although 41 cases presented with cholesteatoma in the retrotympanum, no cases of residual cholesteatoma were found in this area. Residual cholesteatoma was significantly more common in cases with disease of uncertain origin (3 out of 7 vs 2 out of 76; p = 0.004, Fisher's exact test).
Table 1. Summary of characteristics of cases found to have residual disease

No complications were associated with KTP laser use.
Discussion
The sinus tympani is considered an area of high risk for residual cholesteatoma because of inaccessibility for disease removal using traditional microscope-guided approaches. For example, residual disease in the sinus tympani has been reported to be quite common, affecting 19.6 per cent of adults.Reference Glikson, Yousovich, Mansour, Wolf, Migirov and Shapira22 The risk of residual disease in this location may be higher in cases of paediatric cholesteatoma.Reference Jackson, Addison and Prinsley23 We consider that the absence of any residual disease in the retrotympanum in our children with cholesteatoma is likely attributable to careful dissection with the enhanced view provided by endoscopy coupled with the benefit of KTP laser ablation.
We have previously demonstrated reduced residual disease rates in children with smaller cholesteatomas, less intra-operative bleeding and using endoscopes to guide dissection rather than simple inspection after microscope-guided dissection.Reference Thomassin, Korchia and Doris2,Reference Presutti, Marchioni, Mattioli, Villari and Alicandri-Ciufelli3,Reference Marchioni, Alicandri-Ciufelli, Grammatica, Mattioli and Presutti24–Reference le Nobel, Cushing, Papsin and James26
The KTP laser has been shown to reduce the risk of residual cholesteatoma, with a number needed to treat of only four cases (i.e. use of the laser in four cases resulted in one less residual disease case than if the laser had not been used).Reference Hamilton16 This result may be due to devascularisation or ablation of viable keratinocytes left behind during the piecemeal removal of fragments of infiltrative cholesteatoma matrix.Reference Tracy, Kobler, Van Stan and Burns18
Application of the KTP laser in the vicinity of the facial nerve likely poses a risk of facial nerve palsy, even if only temporary.Reference Eskander, Holler and Papsin20 The care taken to avoid this risk may explain the predilection for residual disease in locations close to the nerve in our series. Of note, residual cholesteatoma was also significantly more likely to occur in the small number of cases where the origin of the original disease was uncertain. This category includes cases of possible congenital origin, with no sign of retraction, but with previous myringotomy or perforated tympanic membrane. Such cases often have en plaque disease or bleeding granulation tissue, in which the margins of required dissection are not always obvious. These circumstances likely warrant additional care with disease removal, and attention to second-look or MRI screening findings.
Conclusion
The KTP laser can be used effectively in conjunction with endoscopic dissection to optimise clearance of cholesteatoma from the retrotympanum and other recesses. The attachment of a non-flammable suction cannula to the endoscope facilitates the clearance of smoke plume. Safe application of the technique requires attention to appropriate laser safety measures coupled with careful avoidance of the facial nerve.
Acknowledgement
Grateful thanks to the administrative staff in the ENT department at the Hospital for Sick Children in Toronto, Canada.
Competing interests
None declared