Introduction
Thyroid nodules are common and usually benign. However, prompt evaluation of suspicious nodules is essential to rule out a malignant tumour.Reference Haugen, Alexander, Bible, Doherty, Mandel and Nikiforov1,Reference Marqusee, Benson, Frates, Doubilet, Larsen and Cibas2 Initial physical examination and ultrasonography should be followed by fine needle aspiration if the nodule is large or suspicious for malignancy.Reference Brito, Gionfriddo, Al, Boehmer, Leppin and Reading3,Reference Danese, Sciacchitano, Farsetti, Andreoli and Pontecorvi4 Standard cytology is highly accurate in the diagnosis of most thyroid lesions because of their distinct cellular morphological features.Reference Cibas and Ali5,Reference Bongiovanni, Spitale, Faquin, Mazzucchelli and Baloch6 However, in up to 30 per cent of cases, cytology is not sufficient for a final diagnosis.Reference Ohori and Schoedel7–Reference Alexander, Heering, Benson, Frates, Doubilet and Cibas9 In order to avoid diagnostic surgery, many efforts have been made to develop additional tests that complement standard cytology. Based on known genetic mutations in BRAF, PAX8:PPARγ1 and RET/PTC, molecular testing is now available as an adjunct tool in the decision-making process.Reference Kroll, Sarraf, Pecciarini, Chen, Mueller and Spiegelman10–Reference Ferris, Baloch, Bernet, Chen, Fahey and Ganly15
Fluorescence lifetime is the average time required for a population of excited fluorophores to exponentially decrease by a factor of e, and is in the range of nanoseconds for different fluorophores.Reference Chang, Sud and Mycek16 Fluorescence lifetime is independent of: the intensity of excitation fluorescence, the duration of exposure to light or the concentration of fluorophores.Reference Bastiaens and Squire17 It is, however, sensitive to the internal structure of the fluorophore molecule, and to several external factors such as temperature, pH and polarity. Furthermore, the structural environment of the fluorophores inside living tissue influences fluorescence lifetime.Reference Fixler, Tirosh, Shainberg and Deutsch18,Reference Berezin and Achilefu19
Over the past decades, fluorescence lifetime imaging microscopy has emerged as a complimentary technique to traditional fluorescence intensity measurements in biological research. Fluorescence lifetime imaging microscopy has been used to differentiate between pre-malignant and malignant cells, and to identify the tumour-healthy tissue interface in skin, heart, gastric and oral cavity tissues.Reference Colasanti, Kisslinger, Fabbrocini, Liuzzi, Quarto and Riccio20–Reference Gershanov, Michowiz, Toledano, Yahav, Barinfeld and Hirshberg24
In this research, we explored fluorescence lifetime imaging microscopy in thyroid tissues, and investigated how different thyroid lesions affect fluorescence lifetime for a given fluorophore.
Materials and methods
Patients
The study population included patients who underwent thyroid lobectomy or total thyroidectomy for a multinodular goitre, follicular neoplasms or well-differentiated thyroid cancers. Fresh tissue was collected from the main lesion and immediately frozen to −70°C. Patients were excluded if the lesion was smaller than 1 cm in diameter, or when tissue sampling for this research would have affected the histopathological diagnostic process.
This study was approved by the institutional review board of the Sheba Medical Center, Ramat Gan, Israel. All patients provided written informed consent to participate in this study.
Immunofluorescence staining
The histological diagnosis of the fresh frozen specimens was performed with standard haematoxylin and eosin staining, and confirmed by the pathological report of the entire lesion. As there are no naturally fluorescent molecules specific to thyroid cells, we chose an extrinsic, well-known fluorescent molecule, fluorescein isothiocyanate, and bound it to thyroglobulin.
Immunofluorescent staining was performed with a primary monoclonal mouse anti-human thyroglobulin immunoglobulin G (IgG) antibody (Bio-Rad Laboratories, Hercules, California, USA), and a secondary fluorescein isothiocyanate conjugated goat anti-mouse IgG antibody (Jackson Immunoresearch Laboratories, West Grove, Pennsylvania, USA). The process of immunostaining was performed in a controlled environment, at a temperature of 20 ± 2°C.
Fluorescence lifetime measurements
Fluorescence lifetimes of the stained specimens were measured using a fluorescence lifetime imaging microscopy system (Lambert Instruments, Groningen, The Netherlands). The immunofluorescent stained tissues were repeatedly excited with a sinusoidal 468 nm wavelength. The sinusoidal emitted fluorescent light, peaking at 512 nm, was detected by hybrid photon detectors, and fluorescence lifetime was calculated by LI-FLIM fluorescence lifetime imaging microscopy data analysis software (Lambert Instruments).
Fluorescence lifetime measurements were performed in many different sites in each specimen (50–200 sites per specimen), where fluorescence intensity was visible. In all patients, fluorescence lifetime was measured in cellular areas of the specimens. In the multinodular goitre patients, an additional fluorescence lifetime measurement was performed in the acellular centre of the follicles.
Statistical analysis
The variance and mean fluorescence lifetime was measured for each patient. A repeated measures, mixed model analysis of variance was used to test the difference in fluorescence lifetime between four groups of patients with different thyroid pathologies: multinodular goitre, follicular adenoma, papillary thyroid carcinoma and follicular carcinoma. Comparison between pairs of groups was performed using the student's t-test.
Results
Twelve patients were included in this study: three with multinodular goitre, four with follicular adenoma, four with papillary thyroid carcinoma and one with follicular carcinoma.
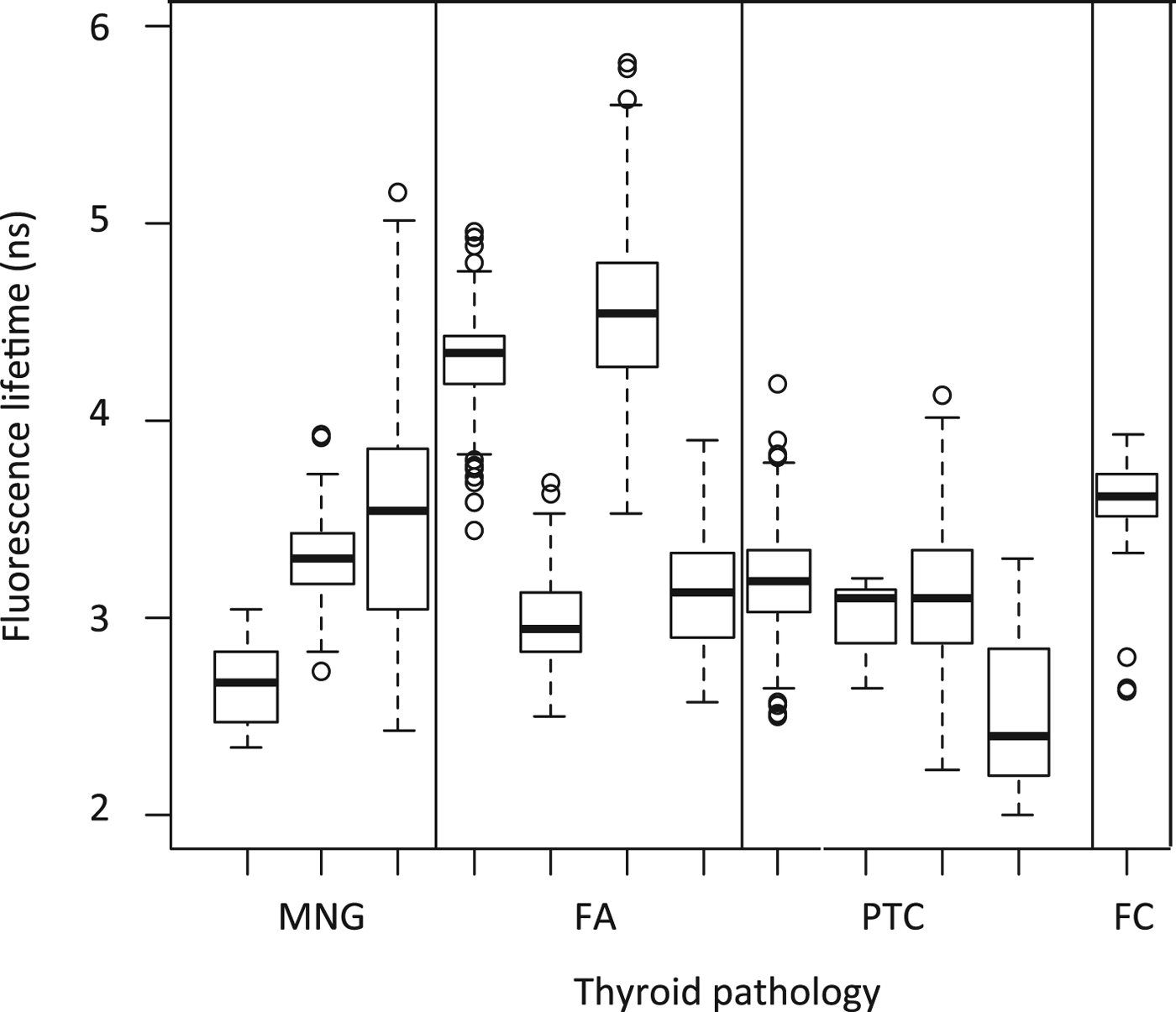
For all patients, a normal distribution of fluorescence lifetime measurements was observed in different sites of the specimen (Figure 1). Fluorescence lifetime of free fluorescein isothiocyanate was 4 ns (range, 3.7–4.3 ns). Fluorescence lifetime of intracellular fluorescein isothiocyanate bound thyroglobulin was significantly lower for all patients, regardless of tissue type.

Fig. 1. Box-and-whisker plot showing fluorescein isothiocyanate fluorescence lifetimes (with outlier values indicated) for individual patients. MNG = multinodular goitre; FA = follicular adenoma; PTC = papillary carcinoma; FC = follicular carcinoma
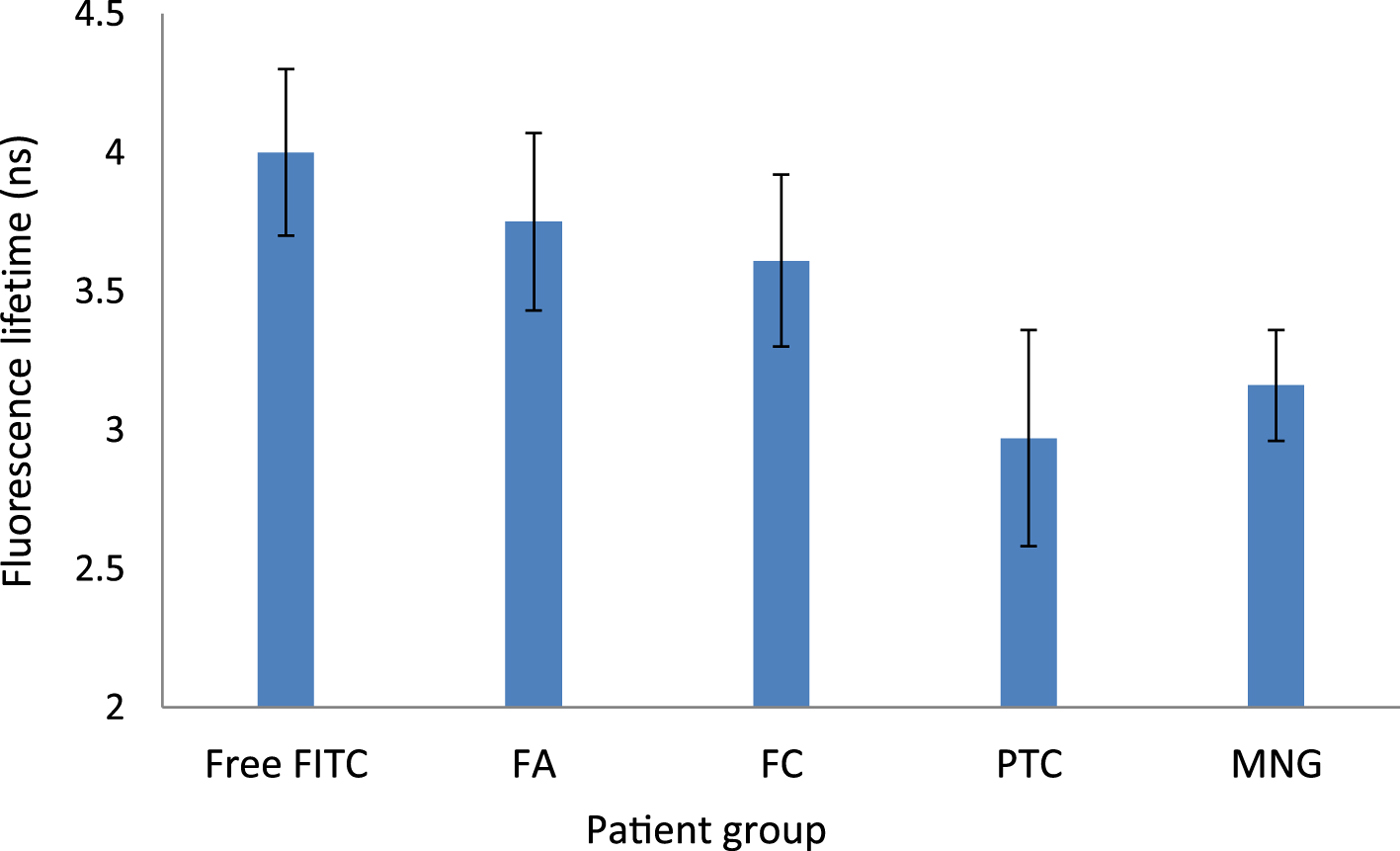
Mean fluorescence lifetime was: 3.16 ns (range, 2.66–3.52 ns) in multinodular goitre patients, 3.75 ns (range, 2.99–4.57 ns) in follicular adenoma patients, 2.97 ns (range, 2.57–3.21 ns) in papillary thyroid carcinoma patients, and 3.61 ns in the follicular carcinoma patient (Figure 2).
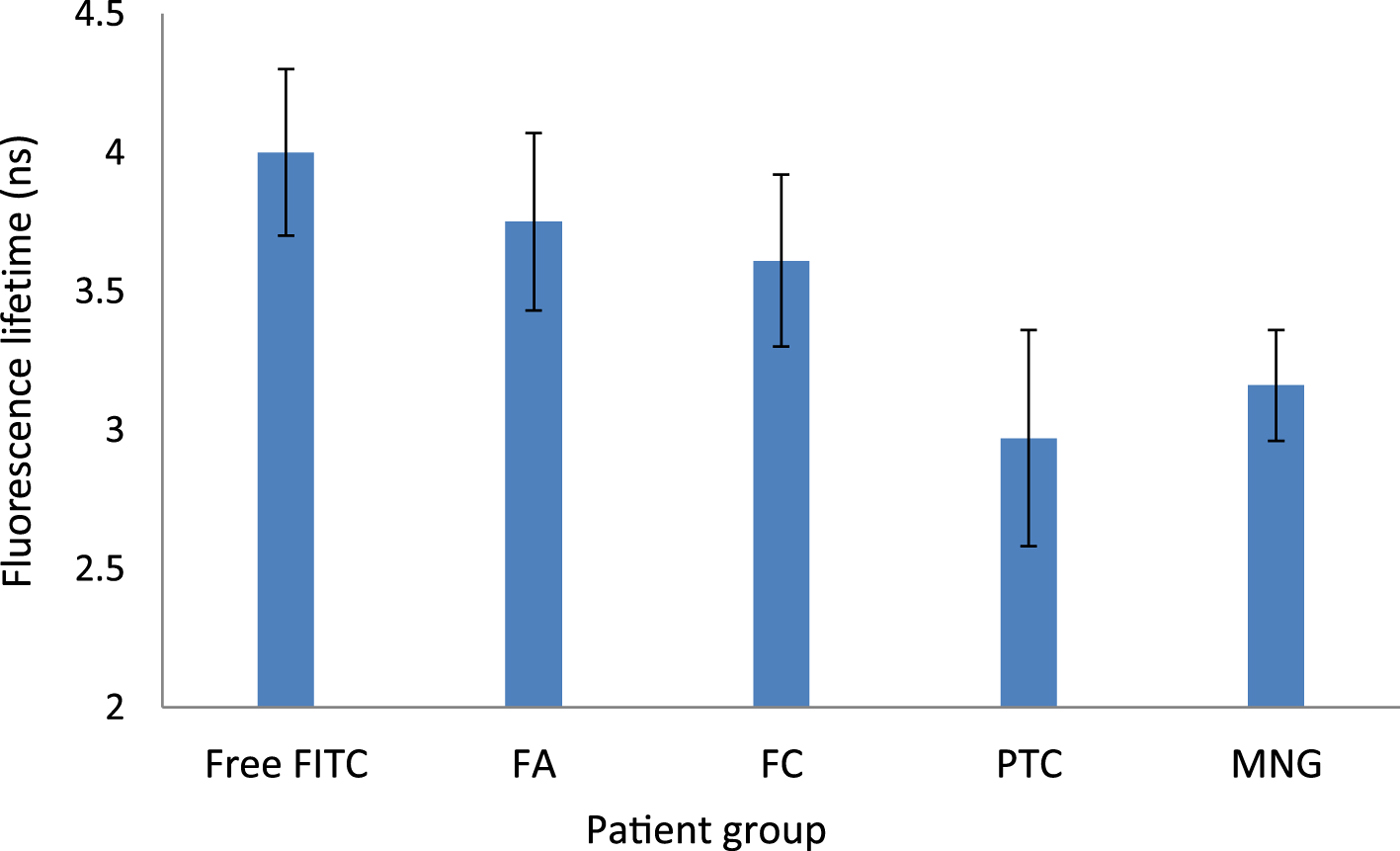
Fig. 2. Mean fluorescein isothiocyanate fluorescence lifetimes (with standard error) for different patient groups. Free-FITC = free fluorescein isothiocyanate; FA = follicular adenoma; FC = follicular carcinoma; PTC = papillary carcinoma; MNG = multinodular goitre
Overall, there was no difference in mean fluorescence lifetime between the groups with multinodular goitre, follicular adenoma, papillary thyroid carcinoma or follicular carcinoma (F (3, 8) = 1.43; p = 0.3). However, a comparison between pairs did reveal differences between the groups. The fluorescence lifetime of follicular adenoma patients was higher than that of papillary thyroid carcinoma patients by 26 per cent, although statistical significance was marginal (p = 0.058). The fluorescence lifetime in the follicular carcinoma patient was similar to that of the follicular adenoma group, but higher than in the papillary thyroid carcinoma group by 22 per cent (p = 0.01).
Discussion
The need for complimentary techniques to differentiate between benign, especially follicular, thyroid lesions and malignant ones has driven the research on the fluorescent properties of thyroid tissues.
Giubileo et al.Reference Giubileo, Colao, Puiu, Panzironi, Brizzi and Rocchini25 analysed the autofluorescence of thyroid surgical samples, and compared follicular cancers with the adjacent healthy thyroid of the same patient. Using synchronous spectral analysis, they found increased intensity of autofluorescence in tumour tissues, as compared to healthy tissues, in two peaks of emission, 340 nm and 390 nm.
Brandao et al.,Reference Brandao, Iwakura, Basilio, Haleplian, Ito and de Freitas26 performed fluorescence lifetime measurements on healthy, benign and malignant thyroid surgical specimens. Fresh thyroid specimens were excited at 285–300 nm light, and fluorescent emission peaks were observed at 340 nm and 450 nm. The authors believed that these emissions represented the endogenous fluorophores elastin and tryptophan (340 nm), and NADH/NADPH (nicotinamide adenine dinucleotide phosphate) (450 nm). Fluorescence lifetime measurements at 340 nm were similar for a healthy thyroid and benign thyroid diseases, such as goitre, follicular adenoma and Hashimoto thyroiditis. In papillary thyroid carcinoma, the fluorescence lifetime was longer than that in healthy or benign thyroid tissues. Such research suggests that the endogenous fluorophores elastin and tryptophan may play a role as thyroid tumour markers.
Our study, with a level of evidence of 4, is probably the first to analyse fluorescence lifetime in fluorescein isothiocyanate bound thyroglobulin, rather than endogenous non-thyroid-specific fluorophores. We chose thyroglobulin, a thyroid-specific protein, as we wanted to explore thyroid cells only, and to exclude other non-thyroidal cells abundant in the tissue. The autofluorescent emissions recorded in other studies, probably representing tryptophan, elastin and NADH, were not specific to thyroid cells. Thyroglobulin is not autofluorescent, and so we had to bind it to a fluorescent molecule, fluorescein isothiocyanate, through a primary and secondary antibody.
In order to evaluate whether this process influenced the fluorescence lifetime of fluorescein isothiocyanate, we measured the fluorescence lifetime of free fluorescein isothiocyanate and extracellular fluorescein isothiocyanate bound thyroglobulin, in the thyroglobulin-rich follicles of multinodular goitre patients. Free fluorescein isothiocyanate and extracellular fluorescein isothiocyanate bound thyroglobulin had similar fluorescence lifetimes, 4 ± 0.3 ns, which is comparable to the known fluorescence lifetime of free fluorescein isothiocyanate, 4.1 ± 0.1 ns.Reference Magde, Rojas and Seybold27
In contrast, our study found that the fluorescence lifetime of fluorescein isothiocyanate bound thyroglobulin was decreased significantly in intracellular environments, regardless of tissue type. There was, however, a difference in the fluorescence lifetimes of different thyroid tissues. Papillary thyroid carcinoma had significantly lower fluorescence lifetimes than follicular carcinoma, and marginally significantly lower fluorescence lifetimes than follicular adenoma. The fluorescence lifetime in multinodular goitre was somewhere in-between that for follicular adenoma/follicular carcinoma and papillary thyroid carcinoma.
We do not know why the fluorescence lifetime of intracellular fluorescein isothiocyanate was lower than that of free fluorescein isothiocyanate, or how tissue type influenced fluorescence lifetime. Temperature and barometric pressure, known extrinsic factors that influence fluorescence lifetime, were monitored and controlled in our study, and were equal in all patients. Other extrinsic factors, such as pH, were not monitored, and might have influenced the fluorescence lifetimes.
• This is the first study to use a thyroid-specific fluorescent-bound marker, fluorescein isothiocyanate bound thyroglobulin, for fluorescence lifetime measurements
• Free fluorescein isothiocyanate and extracellular fluorescein isothiocyanate bound thyroglobulin had the same fluorescence lifetime
• Intracellular fluorescein isothiocyanate bound thyroglobulin had a lower fluorescence lifetime than free fluorescein isothiocyanate or extracellular fluorescein isothiocyanate bound thyroglobulin
• Fluorescence lifetimes of fluorescein isothiocyanate bound thyroglobulin varied in different thyroid pathologies
• This variation in fluorescence lifetimes for multinodular goitre, follicular neoplasms and papillary thyroid carcinoma might be a result of tissue-scale structural influences
As mentioned above, Brandao et al.Reference Brandao, Iwakura, Basilio, Haleplian, Ito and de Freitas26 found that fluorescence lifetime was increased in malignant thyroid tissue, as compared to non-malignant tissue. Our study did not find increased fluorescence lifetimes in malignant lesions; in fact, papillary thyroid carcinoma had the lowest fluorescence lifetimes of all thyroid tissues.
Our cohort was limited by small numbers. Notably, there was only one patient with follicular carcinoma, which is a rare cancer type. Our results, therefore, should be regarded with caution, and not yet considered applicable to the general population.
The main aim of our study was to prove that fluorescent-tagged thyroglobulin could be used as a reliable marker to measure fluorescence lifetime in different thyroid tissues. Our findings might suggest that the fluorescence lifetime of fluorescent-marked cellular thyroglobulin correlates with the architecture of the tissue. Thus, fluorescence lifetimes in follicular carcinoma are similar to the architecturally similar follicular adenoma, but not to papillary carcinoma.
The next step in exploring this new technique should be the measurement of fluorescence lifetime in fluorescein isothiocyanate tagged thyroglobulin of single cells from different thyroid pathologies. Thus, fluorescence lifetime measurements on single cells derived from cytology aspirates where tissue-scale structural influences are absent is the next necessary step towards the application of fluorescence lifetime imaging microscopy to the clinical field.
Competing interests
None declared