Introduction
With the introduction of rigid endoscopes, the endonasal approach to the saccus lacrimalis has become established as a real alternative to external surgical treatment of lacrimal drainage obstruction.Reference McDonogh and Meiring1 Studies of endoscopically performed dacryocystorhinostomy (DCR) report success rates of 81 to 94 per cent, comparable to those of external DCR.Reference Ben Simon, Joseph, Lee, Schwarcz, McCann and Goldberg2,Reference Cho, Paik and Yang3 Therefore, both the endonasal and extranasal approach are well established and widely accepted surgical procedures for the treatment of nasolacrimal duct obstruction. In the hands of otolaryngologists, however, the endonasal endoscopic technique has several advantages, such as allowing paranasal sinus surgery or septoplasty to be performed simultaneously.Reference Horn, Tittmann, Fischer, Otto, Dietz and Mozet4 The avoidance of a facial scar and preservation of the medial canthal ligament are further arguments for choosing an endonasal approach for surgical management of nasolacrimal duct obstruction.Reference Patel5
Even though ENT surgeons are very familiar with navigation-guided endoscopic surgery for paranasal sinus disorders, no studies of image-guided routine endoscopic DCR performed by otolaryngologists exist. Most studies on this topic are by ophthalmologists who have chosen an endonasal endoscopic approach to the saccus lacrimalis with simultaneous use of computed tomography (CT) navigation.Reference Day, Hwang, Pletcher, Bhatki and McCulley6–Reference Ali, Singh and Naik11 Therefore, we conducted a retrospective study of patients with nasolacrimal duct obstruction undergoing routine endoscopic DCR with CT-guided navigation performed by one ENT surgeon in order to evaluate our results from the otolaryngologist's point of view.
Materials and methods
A retrospective study was performed on 16 patients with unilateral or bilateral (n = 1) epiphora who underwent endoscopic DCR with CT-guided navigation between January 2016 and April 2018.
Endoscopic DCR is well accepted as the most suitable treatment for patients with obstructions of the nasolacrimal system, particularly at the level of the lacrimal sac or in the nasolacrimal duct. Thus, in our ENT department endoscopic DCR with CT guidance is the standard procedure for all patients who have a localised obstruction in this area.Reference Penttilä, Smirnov, Tuomilehto, Kaarniranta and Seppä12 Seven patients were male, and the mean age of the study population was 54 years (range, 14–82 years). In most patients, no previous DCR had been performed. Only one patient suffered from recurrence of epiphora after endoscopic DCR without image-guidance that was performed elsewhere. Patients’ characteristics are summarised in Table 1.
Table 1. Patient characteristics

*use of a curved diamond drill; †revision case after previous surgery at another location
All patients or parents provided written informed consent and patients were referred for surgical therapy by ophthalmologists after verification of saccal or post-saccal obstruction by diagnosing reflux during irrigation of the efferent lacrimal duct system. In order to determine the exact location of nasolacrimal duct obstruction and for pre-operative analysis of concomitant nasal pathologies, all enrolled patients underwent low-dose helical CT-dacryocystography with three-dimensional reconstruction. This low-radiation imaging tool, first described in 2002 by Freitag et al., was well tolerated by all patients in our study without pain or complications and provided detailed imaging of the nasolacrimal system including the surrounding structures.Reference Freitag, Woog, Kousoubris and Curtin13
All surgical procedures were performed by one surgeon and under general anaesthesia as an in-patient procedure. During surgery, image-guided dacryolocalisation was performed by means of the BrainLab Kolibri navigation system (BrainLAB, Heimstetten, Germany) after setup and registration with the z-touch system (Figure 1). The calculated registration error was within 1 mm.

Fig. 1. Image showing dacryolocalisation surgery using the BrainLab Kolibri navigation system.
For mucosal decongestion, the nasal cavity was packed with gauze soaked with naphazoline for 5 minutes. After removal of the gauze, an infiltration of the mucosa in the area of the crista lacrimalis in front of the middle turbinate was performed with xylocaine (1 per cent) with adrenalin (1 per cent). Then, a bicanalicular silicone stent (Canaliculus Intubation Set of 15.5 × 0.051 cm; Beaver-Visitec International, Sydney, Australia) with stainless steel probes at both ends was inserted through the upper and lower puncta.
During the next step, a handpiece was used to accurately determine the location of the bony medial wall of the saccus lacrimalis using a 30° rigid endoscope together with the navigation system (Figures 1 and 2). In this area, a small and cranial stemmed mucosal flap was elevated using a sickled knife and a freer elevator in order to minimally expose the bone of the frontal process of the maxilla facing the lacrimal sac (Figure 3). In most cases, a Kerrison punch was used to remove the bone above the medial wall of the lacrimal sac. In three cases, a curved diamond burr was used because of very thick bone in this area. By deeper insertion of one of the steel probes, the medial wall of the lacrimal sac was medialised towards the nasal cavity and incised with a sickled knife. Then, the medial wall of the lacrimal sac was resected and the silicone stent with the two steel probes was pushed into the nasal cavity.

Fig. 2. Image showing precise identification of the bony medial wall of the saccus lacrimalis on the right side using the navigation handpiece.

Fig. 3. Image showing minimal exposure of the bone of the frontal process of the maxilla (white arrow) after elevation of a cranial stemmed mucosal flap (white star).
In the next step, the two ends of the silicone stent were grasped endonasally with small forceps, and the stainless-steel probes were removed. The two ends of the silicone tube were knotted and sutured to the lateral nasal wall. Finally, the mucosal flap was replaced to cover the resilient exposed bone above the lacrimal sac. No nasal package was used, but topical steroid ointment was applied onto the mucosal flap at the end of surgery.
No additional septoplasty or extended paranasal sinus surgery needed to be performed in any patient. However, low-dose CT dacryocystography showed anatomic variations such as hyperpneumatisation of agger nasi cells or concha bullosa in six patients (Figure 4).
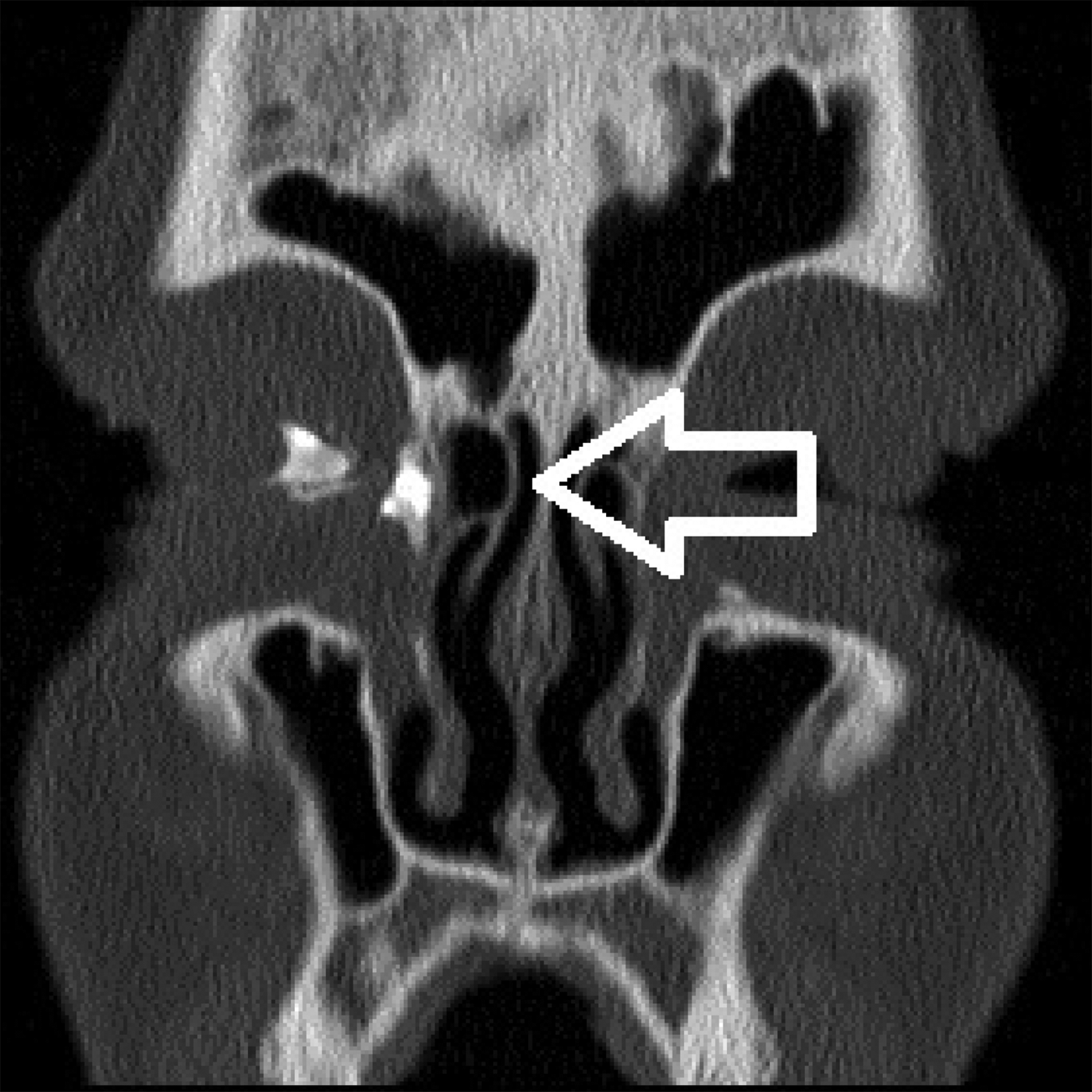
Fig. 4. Image from low-dose computed tomography dacryocystography showing a nasolacrimal drainage obstruction located in the right lacrimal sac. During endoscopic dacryocystorhinostomy, a hyperpneumatised agger nasi cell had to be removed (white arrow).
After surgery, patients underwent regular check-ups for removal of endonasal crusting, which took place either in our department or by an ENT specialist in a private practice. No eye drops or nasal steroid sprays were prescribed. After three months, the silicone tube was removed in our department and patients had two further check-ups at four-week intervals. After removal of the bicanalicular silicone stent, every patient underwent at least two control examinations before final assessment of the result after surgical intervention (minimal follow up of 5 months after endoscopic DCR). We considered our surgery to be successful if the patient was completely symptom-free after the second check-up.
Ethical approval
The present study is retrospective with no changes to any patient's care. Only members of the team caring for included patients had access to non-anonymised data. Data collection was only performed as part of the care of these patients, and all data were collated by the authors in an anonymous form. Therefore, research ethics committee approval was not sought for this retrospective analysis of our results.
Results
By using pre-operative CT-dacryocystography, nasolacrimal system obstruction was precisely located in the lacrimal sac in 10 patients (60 per cent), at the junction of lacrimal sac and nasolacrimal duct in 4 patients (23 per cent) and within the nasolacrimal duct in 3 patients (17 per cent). During all surgical procedures, the location of the lacrimal sac could be accurately determined by navigation (Figure 5), and all 17 endoscopic DCRs were carried out successfully and without intra-operative complications.

Fig. 5. Image showing image-guided navigation to precisely locate the left lacrimal sac before removal of the bone.
Pre-operative setup of the navigation system together with registration was completed within a maximum of 20 minutes in all cases. In two patients, a dislocation of the silicone stent occurred during the early post-operative period (patient 4 in week 3 and patient 11 in week 2; Table 1). In these two cases, the silicone stent was removed transnasally earlier than three months after endoscopic DCR. This procedure succeeded without complications after incising the stent in the area of the lacrimal puncta.
In two patients, a post-operative bacterial infection within the lacrimal sac occurred, causing sanious secretion at the lacrimal puncta (Table 1). Microbiological analysis showed there was infection with Staphylococcus aureus in patient 7 (8 weeks post-operatively) and infection with Streptococcus pneumoniae in patient 10 (5 weeks post-operatively). In both patients, the infection was effectively treated within 1 week using eye drops containing ofloxacin.
In 15 cases, the silicone tube was removed as planned after 3 months (bilateral removal in patient 8). The result of surgical therapy was assessed after the second check-up at 8 weeks after surgery. Patients 4 and 11 also underwent a check-up after 8 weeks, despite earlier removal of the silicone stent. Five months after endoscopic DCR, all patients reported complete remission of epiphora, except for patient 2 (94 per cent). In this case the epiphora continued, and the patient was offered a second surgical intervention. However, the patient refused revision surgery and was referred to her ophthalmologist. Further follow up was performed every 4 weeks in all patients, and after a mean follow up of 7.6 months, the overall success rate was still 94 per cent (complete remission of epiphora).
Discussion
In 1913, West first reported an endonasal technique for DCR, describing resection of the medial wall of the lacrimal sac to treat nasolacrimal duct obstruction.Reference West14 In the next development in 1989, McDonogh et al. introduced endoscopic DCR, a minimally invasive surgical procedure routinely performed by otolaryngologists in particular and now well accepted as an alternative for external DCR.Reference McDonogh and Meiring1 Ophthalmologists have increasingly appreciated the advantages of endoscopic DCR and started to combine endoscopic DCR with CT-guided navigation, particularly in complex endoscopic lacrimal surgical procedures.Reference Day, Hwang, Pletcher, Bhatki and McCulley6–Reference Ali, Singh and Naik11 However, no studies by otolaryngologists examining the benefit of additional CT-guided navigation in routine endoscopic DCR exist.
When clinical examination suggests a mechanical obstruction of the nasolacrimal duct system, the preferred radiological tool for imaging the nasolacrimal system in our ENT department is low-dose CT dacryocystography. Thus, the most important requirement for endoscopic DCR with CT-guided navigation was satisfied in most of our patients suffering from epiphora. Although some authors assume that CT-guidance is a useful adjunct to endoscopic DCR only in challenging or secondary acquired nasolacrimal duct obstruction,Reference Day, Hwang, Pletcher, Bhatki and McCulley6,Reference Ali, Singh, Naik, Kaliki and Dave9,Reference Ali, Singh, Naik, Kaliki and Dave10 we use this technique in all patients undergoing routine endoscopic DCR.
One reason against the use of image-guidance in routine endoscopic DCR might be an increase in operating room time. In our study, set up and registration of navigation took a maximum of 20 minutes, which is comparable to other studies.Reference Metson, Cosenza, Gliklich and Montgomery15 However, those 20 additional minutes significantly enhanced both safety for the patient and comfort for the surgeon. The location of the lacrimal sac could be determined with an accuracy of 1 mm by means of image guidance in all our patients, despite anatomical variations in the nasal cavity, turbinates and nasal septum (Figure 4). As a consequence, maximum preservation of nasal mucosa and bone was possible, contributing to the high success rate of 94 per cent. According to a systematic review by Leong et al., success rates of endoscopic DCR vary between 84 and 94 per cent.Reference Leong, MacEwen and White16 Factors contributing to a high success rate in endoscopic DCR include adequate opening of the bony nasolacrimal canal and adequate removal of the medial wall of the lacrimal sac.Reference Onerci, Orhan, Ogretmenoglu and Irkec17 In our study, navigation guidance significantly facilitated the accomplishment of these two tasks.
Given that stent insertion is helpful for post-operative care and impedes fibrous closure after endoscopic DCR,Reference Bernal-Sprekelsen, Alobid, Tomas-Barberan, Della Rocca, Schaefer, Weber, Keerl, Schaefer and Della Rocca18 we routinely inserted a bicanalicular silicone stent in all patients to be left for three months. However, earlier removal of the stent did not negatively influence the outcome of endoscopic DCR in two of our patients after dislocation of the tube (patients 4 and 11; Table 1). This result is consistent with a systematic review and meta-analysis by Sarode et al., who found no advantage of using a silicone stent in primary endoscopic DCR.Reference Sarode, Bari, Cain, Syed and Williams19 Thus, the efficacy of stent insertion in patients undergoing endoscopic DCR remains uncertain.Reference Nomura, Arakawa, Sugawara, Hidaka, Suzuki and Katori20 Our rate of stent dislocation (12 per cent) is significantly higher than in a study by Horn et al., which reported this post-operative complication after endoscopic DCR in 3 of 102 cases (3 per cent).Reference Horn, Tittmann, Fischer, Otto, Dietz and Mozet4 Both cases of stent dislocation in our study were caused mechanically by the patient drying their face with a towel; therefore, we can rule out an association between our operating technique and the high dislocation rate.
Most of our patients showed a proximal obstruction site in the lacrimal sac (10 patients, 60 per cent of cases), which correlates with a lower success rate for endoscopic DCR in some studies.Reference Nomura, Arakawa, Sugawara, Hidaka, Suzuki and Katori20,Reference Choi, Jin, Moon, Kim, Oh and Kim21 In contrast, Yung et al. reported a success rate of 95 per cent for endoscopic DCR with an obstruction site in the lacrimal sac or duct.Reference Yung and Hardmann-Lea22 In our study, the only patient with persistent epiphora after navigation-guided endoscopic DCR suffered from an obstruction in the lacrimal sac (patient 2; Table 1). Thus, for this location of obstruction, the success rate in our study was 90 per cent. However, in the studies mentioned above, endoscopic DCR was not performed with CT-guided navigation. Therefore, the results cannot adequately be compared with the results of our study, and future studies with a larger study population are needed to analyse the influence of the obstruction site on the success rate of endoscopic DCR with CT-guided navigation.
In a recent retrospective chart review published by Nomura et al., another factor associated with poor prognosis for endoscopic DCR, in addition to a proximal obstruction site, was patient age being 65 years or older.Reference Nomura, Arakawa, Sugawara, Hidaka, Suzuki and Katori20 In our study population, 44 per cent of the patients were 65 years or older. However, in this group of patients our success rate was 100 per cent. As we did not augment the function of the orbicularis muscle that can be weakened due to age, our CT-guided surgical technique cannot be the reason for the high success rate in this subgroup. However, the use of image guidance in our study enabled the surgeon to achieve minimal exposure of bone and maximal preservation of mucosa in all patients, resulting in less bone neogenesis and scaring. Thus, in our opinion, the additional use of navigation guidance can significantly reduce unnecessary collateral damage of tissue surrounding the nasolacrimal duct system, resulting in excellent results after endoscopic DCR in patients of all ages.
• Long-term failure after endoscopic dacryocystorhinostomy (DCR) is most commonly caused by inaccurately locating the lacrimal sac and inappropriate resection of the medial wall of the sac
• Navigation guidance in routine endoscopic DCR accurately targets the precise location and minimises excessive mucosal resection
• This is the first study to evaluate the results of image-guided endoscopic DCR performed by an otolaryngologist
• In this study, the additional use of a navigation system resulted in excellent long-term results after routine endoscopic DCR
• Navigation-guidance can give superior long-term results for surgical treatment of nasolacrimal obstruction, even in ENT departments performing only a few endoscopic DCRs per year
Another factor contributing to the favourable outcomes in our study population may have been the use of a mucosal flap, which has been suggested to decrease cicatricial stenosis after endoscopic DCR.Reference Mueller, Freitag, Lefebvre and Bleier23 The positive effect of this mucosa-sparing technique has been described in previous studies.Reference Mueller, Freitag, Lefebvre and Bleier23,Reference Tsirbas and Wormald24 In our study, an ideal configuration of the flap was maximally facilitated by image-guided dacryolocalisation (Figure 3).
Without routine post-operative application of antibiotic eye drops, two patients in our study developed an infection within the lacrimal sac causing sanious secretion at the lacrima puncta. In a study by Horn et al., all patients routinely received ofloxacin eye drops post-operatively and no infections occurred.Reference Horn, Tittmann, Fischer, Otto, Dietz and Mozet4 Thus, routine post-operative use of antibiotic eye drops might reduce post-operative infection rates. However, whether this procedure has a statistically significant effect needs to be evaluated in future studies with a larger number of patients.
To our knowledge, this is the first study evaluating the results of routine endoscopic DCR with CT-guided navigation performed by otolaryngologists. Our data shows 0 per cent intra-operative complications and 94 per cent success after a minimum follow-up of 5 months, though we only performed 17 endoscopic DCRs over a period of 28 months. A weakness of this study is the lack of a control group without image-guided assistance. However, the success rate of 94 per cent in our patients may indicate that additional use of navigation has a positive effect on the long-term outcome after routine endoscopic DCR.
The strengths of the current study are that we had one single operating surgeon using the same technique in all surgical procedures and a mean follow up of nearly eight months after removal of the silicone intubation. However, this retrospective study is limited by the relatively small number of patients. Therefore, our promising results require confirmation by future prospective studies with larger study populations and a study design that includes patient randomisation to endoscopic DCR with or without the assistance of navigation.
Conclusion
Endonasal endoscopic routine DCR with CT-guided navigation provides additional comfort for the surgeon, maximum safety for the patient and avoids unnecessary damage of bone and mucosa surrounding the lacrimal drainage system. Thus, additional image-guidance may contribute to excellent post-operative results after routine surgical therapy of nasolacrimal duct obstruction.
Competing interests
None declared








