Introduction
This paper focused on a group of neonates with unilateral auditory neuropathy spectrum disorder. We were interested in identifying those with an underlying retrocochlear lesion, such as cochlear nerve aplasia or a cerebellopontine angle tumour.
Auditory neuropathy spectrum disorder is traditionally thought of as a bilateral condition. However, several recent publications have reported cases of unilateral auditory neuropathy spectrum disorder.Reference Zhang, Lan, Shi, Wang, Qi and Zong1, Reference Berlin, Morlet and Hood2 The disorder is characterised by three events. First, cochlear outer hair cell function is preserved. Second, afferent neural transmission from inner hair cells through the auditory nerve to the brainstem pathways is disordered. Third, the efferent feedback mechanism is disordered, as evidenced by absent or abnormal middle-ear muscle reflexes.Reference Berlin, Hood, Morlet, Wilensky, St John and Montgomery3 Normal cochlear outer hair cell function is evidenced by the presence of otoacoustic emissions (OAEs) or a normal cochlear microphonic. Disordered neural transmission is evidenced by absent or abnormal auditory brainstem response (ABR) waveforms.Reference Berlin, Morlet and Hood2
Otoacoustic emissions are acoustic signals that can be recorded with a sensitive microphone positioned within the external auditory canal, and these are thought to reflect the motility of cochlear outer hair cells.Reference Kileny, Zwolan, Flint, Haughey, Lund, Niparko, Richardson and Robbins4 Distortion product OAEs (DPOAEs) are produced following a stimulus to the ear via a miniature loudspeaker (two tones are delivered at 55–85 dB). The mechanism by which the DPOAEs are produced is believed to revolve around both non-linear distortion and linear coherent reflection. These mechanisms are well described elsewhere.Reference Shaffer, Withnell, Dhar, Lilly, Goodman and Harmon5 The DPOAEs can be difficult to detect in the presence of middle-ear effusion, so middle-ear function needs to be assessed in order to interpret the results of DPOAE testing correctly.
The cochlear microphonic is a pre-neural alternating current potential, thought to be summatively generated by the inner and outer hair cells of the cochlea. The outer hair cells are believed to contribute more to this potential, owing to their greater number.Reference Withnell6 It has also been demonstrated in animal models that the cochlear microphonic is dominated by responses from the basal cochlea, within a few millimetres from the round window.Reference Dallos7–Reference Patuzzi, Yates and Johnstone9
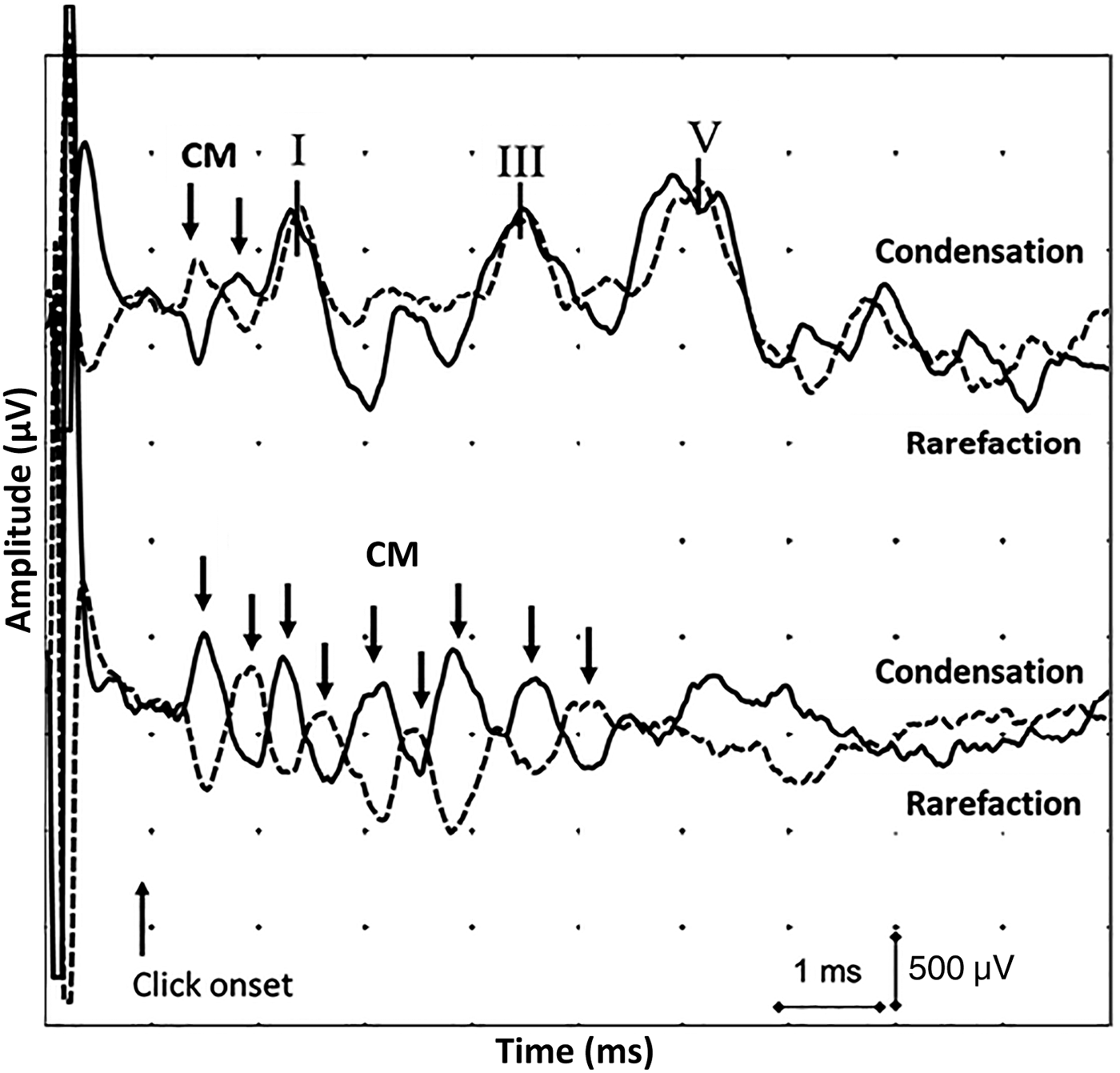
The ABR is an auditory evoked potential generated by neural activity from the auditory nerve and brainstem structures. In a normally functioning auditory system, the cochlear microphonic is usually seen as a single-cycle peak or ‘single ring’, which precedes wave I of the ABR in response to a positive or negative polarity click by 0.5 ms. In the setting of a long-ringing cochlear microphonic, there is evidence of several peaks (Figure 1); this is likely to be due to disordered or absent activation of efferent feedback pathways, which, in normal settings, would suppress outer hair cell oscillation. As a result, the outer hair cells continue to oscillate and the cochlear microphonic is repeated over several cycles.

Fig. 1 Normal (top) and abnormal (bottom) auditory brainstem responses (ABRs) to separate polarity ‘click’ stimuli. The abnormal ABR displays a long-ringing cochlear microphonic (CM) (downward arrows) and absent ABR waveforms. (‘I’, ‘III’ and ‘V’ indicate the respective waves of the ABR.)
The cochlear microphonic can be differentiated from the ABR in three ways: (1) the cochlear microphonic follows the characteristics of the external stimulus – the direction of the cochlear microphonic will reverse with changes in polarity of the stimulus, whereas the ABR will not invert; (2) the cochlear microphonic does not increase in latency as the stimulus intensity decreases; and (3) the cochlear microphonic does not change in latency with masking presented to the ipsilateral ear, while wave I of the ABR shows amplitude reduction and latency increases during simultaneous ipsilateral masking.Reference Lefebvre, Legatt, Van De Water and Hinrich10
This study aimed to investigate whether the aetiology for hearing impairment in neonates with unilateral auditory neuropathy spectrum disorder could be explained by structural abnormalities such as cochlear nerve aplasia, a cerebellopontine angle tumour or another identifiable lesion.
Materials and methods
All neonates in the northern New South Wales region (Australia) who are identified by the Statewide Infant Screening – Hearing (‘SWISH’) programme as having a hearing loss are currently referred for diagnostic audiology at John Hunter Children's Hospital, New Lambton Heights. This further assessment consists of distortion product OAE (DPOAE) testing, tympanometry and ABR testing (with an output up to 95 dB). For the current study, we prospectively enrolled neonates with unilateral auditory neuropathy spectrum disorder, identified on electrophysiological testing.
Diagnostic audiometry was performed in a standardised manner with the use of the Natus Bio-logic Navigator Pro diagnostic ABR system (Natus Medical, San Carlos, California, USA). The system is used with Master II® software to calculate and analyse DPOAEs, cochlear microphonics and ABRs, via click and tone burst stimuli.
The ABR testing was conducted using intensities of 30–90 dB in order to determine hearing thresholds. Testing was initially performed with click stimuli. Tone burst stimuli were subsequently used at 1, 2 and 4 kHz.
Our entry criteria for the study were a unilateral diagnosis of OAE and/or a long-ringing cochlear microphonic, with abnormal ABRs, as per Berlin and colleagues.Reference Berlin, Morlet and Hood2, Reference Berlin, Hood, Morlet, Wilensky, St John and Montgomery3 There are no traditional guidelines for the length of a cochlear microphonic in determining a long-ringing cochlear microphonic. We deliberately allowed relatively wide-field entry criteria in order to avoid the type II error of missing a serious lesion, including cerebellopontine angle tumours.
The neonates were assessed in our Baby Hearing Clinic, where details of patient history, physical examination findings, pathology and imaging findings were collected. Data were recorded in an Excel spreadsheet (Microsoft, Redmond, Washington, USA) for subsequent analysis.
Results
Audiology
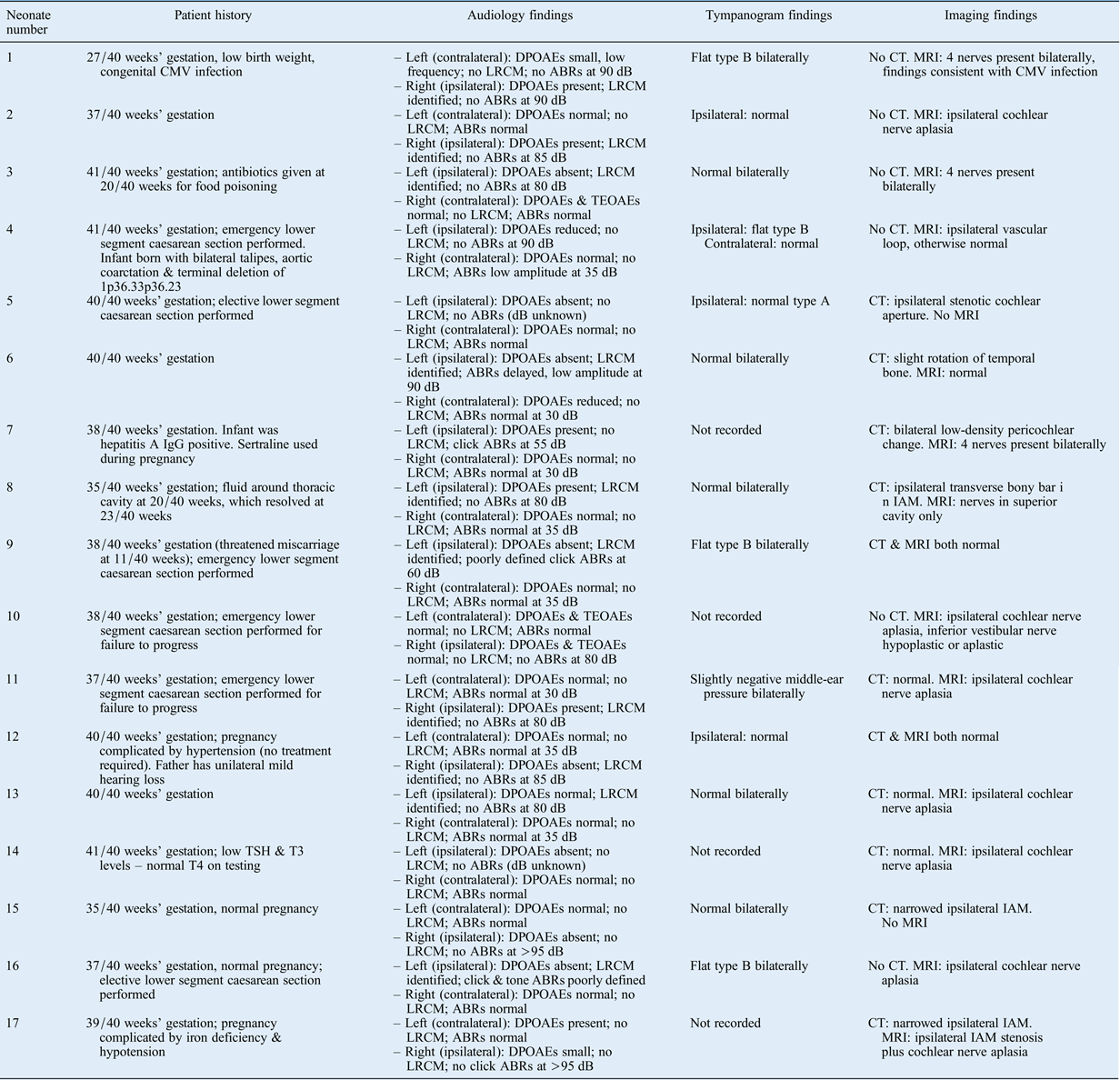
Seventeen neonates (10 males and 7 females) with evidence of unilateral auditory neuropathy spectrum disorder were identified. The findings are summarised in Table I. Auditory brainstem response waveforms in the affected ear were completely absent in 13 infants, delayed in 1, elevated in 1 and poorly defined in 2. Distortion product OAEs were present in seven infants, reduced in two and absent in eight. A long-ringing cochlear microphonic was identified in 10 infants.
Table I Patient history, audiology and imaging findings*
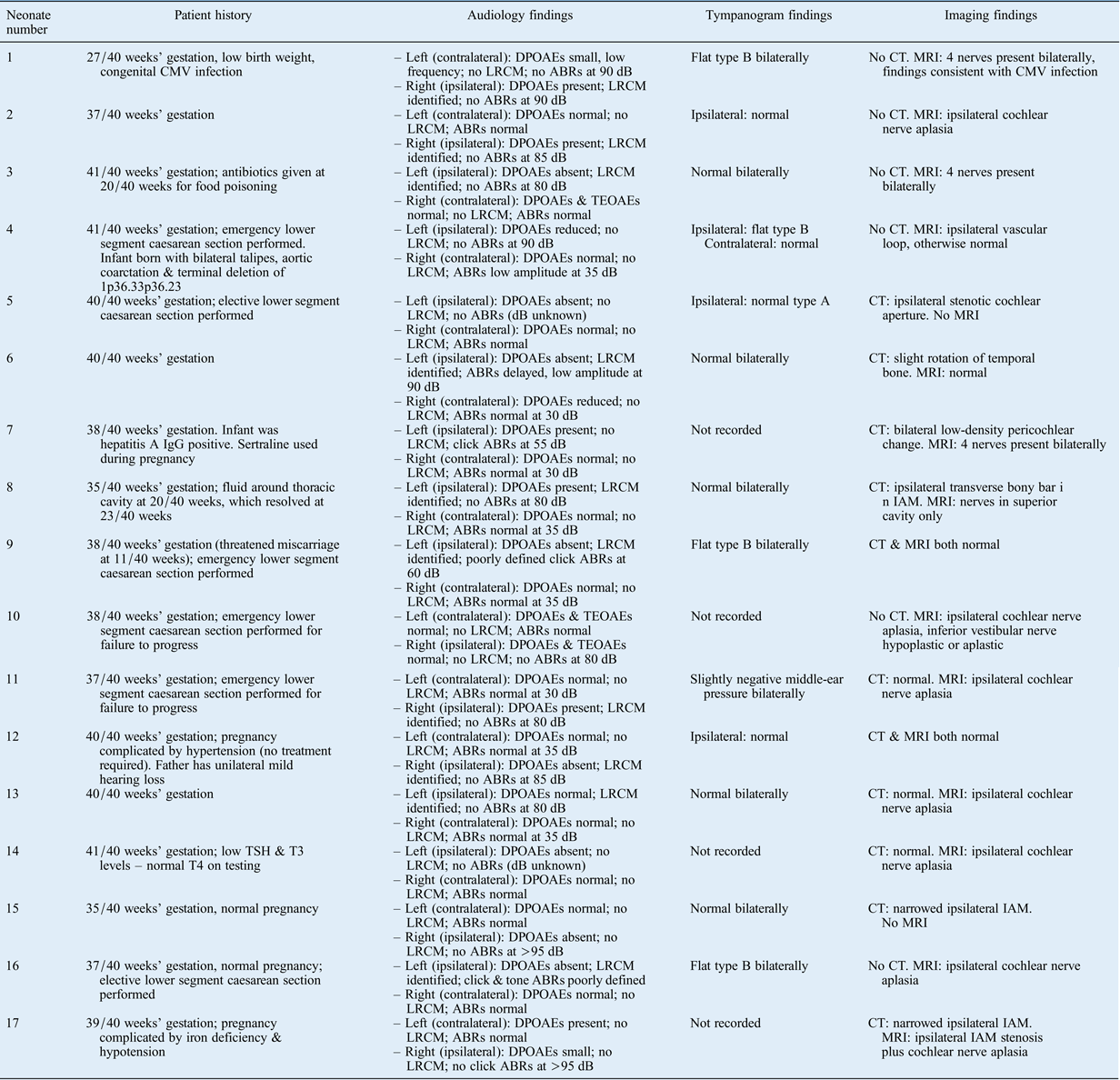
* For 17 neonates with unilateral auditory neuropathy spectrum disorder. CMV = cytomegalovirus; DPOAE = distortion product otoacoustic emission; LRCM = long-ringing cochlear microphonic; ABR = auditory brainstem response; CT = computed tomography; MRI = magnetic resonance imaging; IgG = immunoglobulin G; TEOAE = transient evoked otoacoustic emission; TSH = thyroid-stimulating hormone (thyrotropin); T3 = triiodothyronine; T4 = thyroxine; IAM = internal auditory meatus
Imaging
Eleven neonates underwent computed tomography (CT) and 15 underwent magnetic resonance imaging (MRI). Two infants underwent CT alone, six underwent MRI alone, and nine underwent both CT and MRI.
Of the 11 neonates who underwent CT scanning, abnormalities ipsilateral to the ear with auditory neuropathy spectrum disorder were recognised in 6 infants. Abnormalities on CT included a narrowed internal auditory meatus (IAM) (n = 3), a transverse bony bar in the IAM (n = 1), slight rotation of the temporal bone (n = 1) and low-density pericochlear change (n = 1). The five remaining infants had a normal CT, including a normal-calibre IAM. Three of these infants were subsequently shown, on MRI, to have aplasia of the cochlear nerve.
A CT finding of a stenosed IAM raised a suspicion of cochlear nerve aplasia or hypoplasia. Of the three neonates with a narrowed IAM, MRI results were available for one infant, which confirmed ipsilateral cochlear nerve aplasia. Magnetic resonance imaging was not performed on the remaining two infants: one family did not attend follow up and the other family declined MRI in order to avoid general anaesthesia.
Of the 15 neonates who underwent MRI, abnormalities were recognised in 10 infants. Abnormalities on MRI included: cochlear nerve aplasia (n = 8); a vascular loop created by the anterior inferior cerebellar artery, abutting the vestibulocochlear complex (n = 1); and evidence of in utero cytomegalovirus infection (n = 1). All of these patients had non-recordable ABRs below 80 dB.
Ten neonates showed evidence of cochlear nerve aplasia on CT or MRI, or both. Eight were definitively confirmed, via MRI, to have cochlear nerve aplasia (Figures 2a and 2b). One of these was shown on CT to have a transverse bony bar in the IAM. The MRI in this neonate demonstrated nerves within the superior cavity only. The identified transverse bar may represent an extension of the falciform crest.

Fig. 2 Sagittal magnetic resonance imaging scans for neonate number two, showing: (a) a normal cochlear nerve (arrow) situated within the left internal auditory meatus (IAM), and (b) cochlear nerve aplasia in the contralateral (right) IAM.
In this series of 17 neonates, all of whom had electrophysiological findings consistent with unilateral auditory neuropathy spectrum disorder, 10 (59 per cent) showed evidence consistent with cochlear nerve aplasia ipsilateral to the audiometric findings. Another abnormality was revealed by MRI or CT in four neonates. There were only three infants (18 per cent) in the series in whom cochlear nerve aplasia or another lesion was not found. Computed tomography missed three cochlear nerve aplasias; these were confirmed on MRI. The MRI scans provided a definitive diagnosis in eight of the infants. Thus, we suggest that MRI is a necessary first-line imaging modality.
Discussion
Auditory neuropathy spectrum disorder is not a rare disorder. According to two recent papers, 5 per cent of all children are affected, and the prevalence rises to 14 per cent in children diagnosed with severe to profound sensorineural hearing loss.Reference Bielecki, Horbulewicz and Wolan11, Reference Mittal, Ramesh, Panwar, Nilkanthan, Nair and Mehra12 The prevalence of the disorder in newborn populations was determined by the use of OAEs in those studies. Recently, Kirkim et al. found that the prevalence of the disorder was close to 0.044 per cent for the total population when prevalence was determined via a universal newborn hearing screening programme with the aid of automated ABR testing.Reference Kirkim, Serbetcioglu, Erdag and Kerim13 This represented 15.38 per cent of 65 patients with abnormal ABR results.
It is important to identify and then investigate the cause of unilateral auditory neuropathy spectrum disorder in order to provide families with the most appropriate recommendations regarding management. The results reported here suggest that in 10 neonates (59 per cent of the series), the underlying cause was ipsilateral cochlear nerve aplasia. This finding underlines the need for further investigation once diagnosis of the disorder has been made.
A long-ringing cochlear microphonic, which would be expected consequent to the absence of efferent feedback, was not always identified. In this study, a long-ringing cochlear microphonic was identified in 10 infants (59 per cent), with the remaining 7 infants displaying evidence of a (non long-ringing) cochlear microphonic. It is possible that a long-ringing cochlear microphonic is dependent on some prerequisite neural response. However, evidence in this area is lacking. The finding suggests that patients with cochlear nerve aplasia, as visualised on MRI, do not necessarily have complete loss of auditory innervation of the cochlea. Although more evidence is required, at least one small study from 2010 showed responses to auditory stimuli following cochlear implantation in children.Reference Warren, Wiggins, Pitt, Harnsberger and Shelton14 In other children with cochlear nerve aplasia, an auditory brainstem implant may be a feasible option.Reference Colletti, Carner, Fiorino, Sacchetto, Miorelli and Orsi15 There is much that is not known or understood about neonates with cochlear nerve aplasia requiring implantation; this group would thus benefit from further investigation.
Computed tomography scanning has the advantage of requiring only sedation, rather than general anaesthetic. It is, however, associated with exposure to radiation. Magnetic resonance imaging is non-ionising, but requires a general anaesthetic. Computed tomography scanning is more readily available in our institution than MRI performed under general anaesthesia. In order to avoid multiple visits for families living remotely, CT is provisionally booked to follow confirmation of hearing loss during diagnostic audiology, with same-day or next-day review in our Baby Hearing Clinic. This approach is currently under review. Families should be counselled about the pros and cons of each modality. In this series, MRI was definitive.
Recent papers have shown that MRI assessment, especially evaluation of the cochlear nerve, can predict the viability and success of cochlear implantation in auditory neuropathy spectrum disorder neonates with hypoplastic or aplastic cochlear nerves.Reference Jeong and Kim16, Reference Walton, Gibson, Sanli and Prelog17 Walton et al. demonstrated that auditory neuropathy spectrum disorder patients with cochlear nerve deficiency had worse speech perception scores at one year of age than those without cochlear nerve deficiency.Reference Walton, Gibson, Sanli and Prelog17 This was demonstrated again by Jeong et al. in their 2013 study.Reference Jeong and Kim16
• Auditory neuropathy spectrum disorder is traditionally thought of as a bilateral condition, but several unilateral cases have been described previously
• In the current study, 17 neonates showed evidence of unilateral auditory neuropathy spectrum disorder
• Further investigation demonstrated cochlear nerve aplasia in 10 neonates, another abnormality in 4 and no abnormality in only 3
• Computed tomography missed three cochlear nerve aplasias; these were confirmed on magnetic resonance imaging
• Magnetic resonance imaging should be the first-line imaging modality in neonates with evidence of unilateral auditory neuropathy spectrum disorder
Our results show that CT can miss cochlear nerve hypoplasia or aplasia as compared to MRI. Three neonates who underwent CT and MRI demonstrated cochlear nerve aplasia only on MRI, with CT findings being reported as normal. This is also noted in the paper by Adunka et al., in which the authors recommended MRI for the investigation of patients with severe to profound sensorineural hearing loss.Reference Adunka, Jewells and Buchman18
Conclusion
The results reported here demonstrate that a suspicion of unilateral auditory neuropathy spectrum disorder mandates imaging in order to establish a definitive diagnosis. In this series of 17 neonates, only 3 (18 per cent) did not demonstrate any abnormality on MRI or CT. Ten infants (59 per cent) showed evidence of cochlear nerve aplasia. In three infants, MRI scans showed cochlear nerve aplasia with no corresponding evidence on CT scans. Magnetic resonance imaging is justified as a first-line investigation in the diagnostic assessment of unilateral auditory neuropathy spectrum disorder findings.
Acknowledgements
The authors are grateful to Dr Larry Roddick and David Gosling for their contributions in the clinical and audiological examinations of the neonates.