Introduction
Epistaxis is a common condition presenting to otolaryngologists, affecting around 10 per cent of the general population.Reference Shaheen, Kerr and Groves1 It occurs more frequently in children. Previous research has shown epistaxis to affect up to 30 per cent of children aged zero to five years, 56 per cent of those aged six to 10 years and 64 per cent of those aged 11 to 15 years.Reference Petruson2 It is also known that 56 per cent of adults suffering from recurrent epistaxis first began to experience problems during childhood.Reference Beran and Petruson3
For more than 90 per cent of all patients with epistaxis, the source of bleeding can be identified in the anterior septal mucosa,Reference Josephson, Godley and Stierna4 making it a relatively easy condition to treat. The use of nasal creams and ointments has been widely described in the treatment and management of anterior epistaxis,Reference Josephson, Godley and Stierna4–Reference Leong11 and is usually the preferred treatment option for children with mild epistaxis. Such topical treatments are also frequently used as adjunctive therapy following silver nitrate cautery or diathermy.
There remains a great degree of variation in the choice of topical agents however, and a brief examination of practice at our institution confirmed that such variation was not limited to the choice of cream or ointment, but also extended to the associated instructions given to patients. We reviewed the literature in an attempt to find established evidence on which basis to improve and standardise our practice. However, as is the case for much everyday otolaryngology practice, there was a lack of hard data.
Although some studies have attempted to compare such topical treatments with placebos,Reference Kubba, MacAndie, Botma, Robinson, O'Donnell and Robertson5, Reference Loughran, Spinou, Clement, Cathcart, Kubba and Geddes6 no two topical treatments have been compared with each other as yet. Therefore, the findings remain inconclusive, with a ‘gold standard’ topical treatment yet to emerge.
The aim of this survey was to determine the current prevalent practice regarding the use of creams and ointments in the management of anterior epistaxis. By establishing the data required to compare and to further study variations in such treatment, we aimed to strengthen the foundations for evidence-based practice regarding this common ENT ailment.
Methods
A comprehensive list of all Scotland-based ENT consultants and trainees registered with ENT-UK was obtained. This list comprised a total of 105 clinicians. A questionnaire (Appendix 1) was then devised and posted out, together with a covering letter explaining the reasons for the survey. Responses were awaited for a period of two months. Once received, these responses were entered into Excel spreadsheets and then further analysed. Respondents who stated they did not use any treatment were excluded from further analysis.
Results
Of the 105 participants, 53 per cent were consultants (n = 56) and 47 per cent were trainees (n = 49). A total of 95 responses were received, with an 82 per cent response from consultants and a 100 per cent response from trainees, giving an overall response rate of 91 per cent. These data are shown in Figure 1.
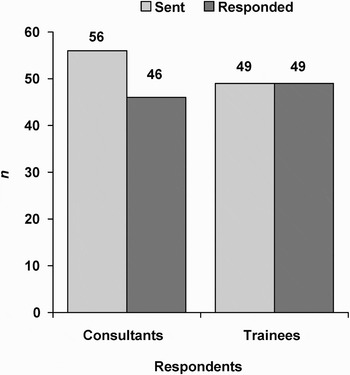
Fig. 1 Survey response rates.
Although only one answer was required per question, many respondents selected more than one. This was probably representative of the fact that, nationally, no one treatment is currently considered best; and therefore, no set standards exist. All selected answers were therefore considered in the further analysis; as a result, where percentages are presented, the total is frequently over 100 per cent.
The most popular topical agent for the management of anterior epistaxis was Naseptin® (Alliance Pharmaceuticals Ltd, Wiltshire, UK). Eighty-seven per cent of overall respondents selected this preparation, with 83 per cent of consultants and 92 per cent of trainees choosing it as their preferred topical treatment. The use of petroleum jelly (Vaseline®) and Bactroban (GlaxoSmithKline, Middlesex, UK) was minimal, with 9 and 5 per cent of respondents opting for them, respectively. Data on respondents' choice of treatment is shown in Figure 2. Four per cent of respondents indicated that they recommend no topical treatment for epistaxis; these respondents were subsequently excluded from further analysis.
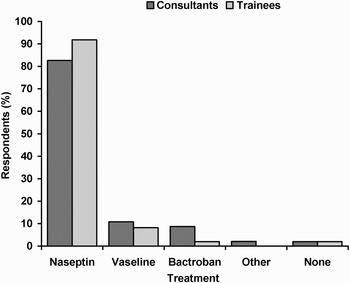
Fig. 2 Respondents' preferred treatment type.
Overall, the most commonly advocated frequency with which to apply treatment was twice a day (46 per cent), followed by three times a day (35 per cent). When analysed separately, this preference was similar for the consultant group, however, trainees appeared to favour application three times a day (43 per cent) over twice a day (37 per cent). Four respondents recommended applying the treatment four times a day and two advocated applying it ‘PRN’ (i.e. as required). Data on respondents' preferred frequency of use are shown in Figure 3.

Fig. 3 Respondents' preferred treatment frequency.
The most popular recommended application methods for topical treatment were either by inserting the nozzle of the tube into the nostril (57 per cent), or direct application using the patient's own finger (32 per cent). Half of the consultants recommended nozzle application and 37 per cent preferred finger application. A higher proportion of trainees, 63 per cent, opted for nozzle application, with only 27 per cent of trainees suggesting patients use their own finger. Thirteen per cent of respondents recommended application using a cotton bud (‘Q-tip’), and one even described their own method of application using the handle of a teaspoon. Data on respondents' preferred methods of application are shown in Figure 4.

Fig. 4 Respondents' preferred method of application.
The majority of respondents (45 per cent) indicated that they suggested a two-week duration of treatment. This figure was comparable between both groups, with 48 per cent of consultants and 43 per cent of trainees preferring this treatment duration. Seventeen per cent of consultants suggested a one-week treatment duration, and 18 per cent of trainees favoured a one-month duration. Eighteen per cent of respondents recommended various other treatment durations, ranging from 10 days to six weeks. Using the treatment on a ‘PRN’ basis was also suggested, as was continuing the therapy until the tube was empty. Data on respondents' preferred treatment duration are shown in Figure 5.

Fig. 5 Respondents' preferred treatment duration. Wk = weeks; mth = month
Discussion
This Scottish national survey demonstrated considerable variation in practice in the use of nasal preparations. While several previous studies have attempted to address some of the questions asked, the majority of these studies have been based on paediatric cohorts, and their results remain inconclusive. A Cochrane database systematic review carried out by Burton and Doree in 2005 concluded that the optimal management for children with recurrent, idiopathic epistaxis was unknown.Reference Burton and Doree8
In the absence of established evidence, clinicians are usually required to call upon their own judgement and relative expertise in order to make appropriate management decisions. However, there are a number of other factors which should be kept in mind when using topical creams or ointments to treat epistaxis.
Nasal ointments and creams act by reducing the drying and crusting of nasal mucosa, with some also providing antiseptic and/or antibiotic properties in addition. The effect of this is a reduction in vestibulitis and further epistaxis. A randomised, controlled trial by Kubba et al. demonstrated the use of Naseptin to be superior to no topical treatment,Reference Kubba, MacAndie, Botma, Robinson, O'Donnell and Robertson5 and other studies have shown Naseptin to be just as effective as silver nitrate cautery.Reference Ruddy, Proops, Pearman and Ruddy9 However, Loughran et al., using a randomised, controlled trial, found that the use of petroleum jelly had no benefit over simple observation.Reference Loughran, Spinou, Clement, Cathcart, Kubba and Geddes6 We note that both studies were performed on a paediatric population.Reference Kubba, MacAndie, Botma, Robinson, O'Donnell and Robertson5, Reference Loughran, Spinou, Clement, Cathcart, Kubba and Geddes6
Of all the products mentioned above, Naseptin and Bactroban are the only two licensed for intranasal use. Their use is advocated for the prophylaxis and treatment of intranasal infection and, in particular, for nasal carriage of methicillin-resistant Staphylococcus aureus. Neither of these products is licensed for use in epistaxis.
Naseptin cream comprises an antiseptic and an antibiotic, namely, chlorhexidine hydrochloride and neomycin sulphate. The active ingredient in Bactroban ointment is mupirocin in a white soft paraffin base. Petroleum jelly is a non-medicinal emolient which is readily available over the counter.
The instructions accompanying Naseptin advise that it should be applied twice a day for the prevention of, and four times a day for the treatment of, nasal staphylococcal carriage, for a duration of 10 days. Bactroban is advised to be used two to three times a day, and for no longer than 14 days. Petroleum jelly is a non-medicinal product so there is no recommended guidance for its intranasal use.
Usage may also be influenced by price, as there are significant price differences between some of the treatments mentioned by our respondents. Obtaining retail prices from the British National Formulary,12 the biggest price difference appears between the two most popular choices, with Naseptin costing £1.58 for 15 g while Bactroban costs £5.80 for 3 g. Price differences may in turn influence the availability of the treatment, as some hospital pharmacies may favour and stock only the cheaper options.
A clear history of an allergy to any of the constituents will obviously influence the clinician's decision on choice of treatment. One of the better known excipients includes arachis oil, which is a peanut extract and present in Naseptin. While there is insufficient evidence to support the theory that exposure of inflamed mucosa to topical medicines containing peanut extracts may lead to sensitisation,Reference Lack, Fox, Northstone and Golding13 the 2003 Chief Medical Officer's Update cautioned against using such products in patients with known peanut or soya allergies.Reference Donaldson14
• The use of nasal creams and ointments in the conservative management of anterior epistaxis is well documented and supported
• This study set out to obtain a national opinion, in order to establish current practice
• This national survey indicated considerable variation in practice regarding the use of topical nasal treatments
• In the absence of established evidence, clinicians are usually required to call upon their own judgement and relative expertise in order to make appropriate management decisions
Information regarding the method of application of the above-mentioned creams and ointments is limited. The information leaflet accompanying Naseptin suggests using the tip of the patient's little finger, while the Bactroban information leaflet suggests the same method or, alternatively, using the end of a cotton bud. Petroleum jelly is not a medicinal product for nasal treatment, and hence there are no recommendations regarding methods for its application.
Age must also be considered. If the patient is a young child, the parents will need to take on the role of applying the treatment. Using the nozzle of the tube can allow the user to control the direction of the application, but the tip does have the ability to traumatise sensitive nasal mucosa if care is not taken, and blind application does not enable measurement of the quantity of treatment applied. Those applying the treatment with a cotton bud will also run a similar risk of trauma, as the applicator will come into direct contact with the mucosa. Badran and Jani recently described a novel method of using a nozzle but making it potentially less traumatic by using a soft rubber tip at the end.Reference Badran and Jani7 Using a cotton bud or a finger tip may allow the user to quantify the amount of treatment being used; however, patients using their fingers to apply the treatment should be advised to keep their nails cut short and to use only the pulp of the finger. However, clinicians should also bear in mind that a large proportion of patients with epistaxis tend to be older, and therefore that simpler more straightforward methods may perhaps be more appropriate in this age group, as highlighted in another recent paper.Reference Leong11
Study limitations
A survey of any kind is not designed to provide high level evidence regarding the study question but rather to depict current trends and practice. The latter was our aim when designing this survey, and we believe that we succeeded, within the limitations mentioned below.
The response rate was 91 per cent, which is considered a very respectable response rate for a survey. Our survey was conducted at a national level, using database information obtained from ENT-UK. Although most otolaryngologists are registered with ENT-UK, we accept that the list obtained was in all probability incomplete. No attempt was made to trace clinicians not included in the provided list.
Conclusions
The use of nasal creams and ointments is widely accepted and advocated, and they are often the mainstay of treatment in the conservative management of anterior epistaxis. This survey clearly demonstrated considerable variation in respondents' preferred choice of topical agent, as well as in their preferred treatment frequency, duration and application method.
This survey endeavoured to obtain a national opinion and consensus regarding these variations. Our results demonstrate that the majority of ENT clinicians across Scotland favour the use of Naseptin, applied using the nozzle of the tube, for a period of two weeks. Consultants tend to favour application twice a day, while trainees appear to favour application three times a day.
We believe that this survey successfully indicates current prevalent practice and provides a benchmark for comparison of individual practices. Our results demonstrate the variations in current practice and highlight the need for further, definitive research in order to establish an evidence-based gold standard topical treatment for anterior epistaxis.
Appendix 1. Survey questionnaire
Regarding the use of nasal creams and ointments in patients with anterior epistaxis:
Which cream or ointment do you use most commonly?
Naseptin □
Bactroban □
Vaseline □
Other □
None (stop here) □
How often do you ask your patient to use the treatment?
Once a day □
Twice a day □
Three times a day □
Other …
How do you usually advise your patients to use the treatment?
Inserting the nozzle into the nostril □
Applying with cotton bud / Q-tip □
Applying with finger □
Other …
How long do you ask your patients to use the treatment for?
One week □
Two weeks □
Three weeks □
One month □
Other …







