Introduction
Although many mechanisms of noise-induced hearing loss have been defined, its pathogenesis has not yet been completely elucidated. The primary factor in noise-induced hearing loss is apoptosis in the Organ of Corti.Reference Mulroy, Henry and McNeil1 The main causes of cell death following acoustic trauma are cited as diminished blood flow to the inner ear,Reference Miller, Ren, Dengerink, Nuttall, Axelsson, Borchgrevink, Hamernik, Hellstrom, Henderson and Salvi2 production of free radicals due to enhanced metabolic activity,Reference Ohlemiller, McFadden, Ding, Lear and Ho3 and cellular necrosis of the outer hair cells, induced directly by mechanical trauma.Reference Saunders, Dear and Schneider4 Following acoustic trauma, free oxygen radicals are formed in the inner ear.Reference Miller, Yamashita, Minami, Yamasoba and Le Prell5 These substances damage the cells by: altering the structure of cellular DNA;Reference Van Campen, Murphy, Franks, Mathias and Toraason6 injuring the cellular membrane through lipid peroxidation;Reference Ohinata, Miller, Altschuler and Schacht7 and diminishing cochlear blood flow.Reference Miller, Brown and Schacht8
While free oxygen radical production plays an important role in the pathogenesis of various neurodegenerative syndromes, antioxidant agents might have a therapeutic role in neurodegenerative processes.Reference Casetta, Govoni and Granieri9 Thus, the use of antioxidants may be beneficial in treating noise-induced hearing loss, which itself is a neurodegenerative process. The increase in free oxygen radical production may continue for up to 7–10 days after noise exposure; the duration of that period depends on the type of noise parameters and their variety.Reference Yamashita, Jiang, Schacht and Miller10 Following exposure to noise, hydroxyl radicals and superoxides increase during the early stages (after 1–2 hours).Reference Yamane, Nakai, Takayama, Iqucgi, Nakaqawa and Kojima11 The free oxygen radicals formed in the first 1–30 minutes following noise exposure are lipid peroxidation products and peroxynitrite,Reference Henderson, Bielefeld, Harris and Hu12 and lipid peroxidation products keep increasing in the first 1–5 hours.Reference Ohinata, Miller, Altschuler and Schacht7
Nigella (Nigella sativa L), a member of the Ranunculaceae family, is a herbaceous plant native to Mediterranean countries.Reference Ramadan13 It is composed of 36–38 per cent fixed oils, proteins, alkaloids and saponins, and 0.4–2.5 per cent essential oils. Analysis of its essential oils using high performance liquid chromatography has shown that it contains thymoquinone, dithymoquinone, thymohydroquinone and thymol.Reference Arslan, Gelir, Armutcu, Coskun, Grel and Sayan14 Thymoquinone (2-isopropyl-5-methyl-1,4-benzoquinone; molecular formula, C10H12O2) is the major bioactive molecule of N sativa.Reference Dehkordi and Kamkhah15 It possesses antioxidant, anti-tumour, anti-inflammatory, antibacterial, antiviral, immunomodulatory, antihistaminic, antihelmintic and anti-aggregant properties.Reference Vanamala, Kester, Heuberger and Reddivari16
Thymoquinone implements those effects through the inhibition of cyclo-oxygenases, lipoxygenases and membrane lipid peroxidation.Reference Hosseinzadeh, Parvardeh, Asl, Sadeqhnia and Ziaee17 It exhibits a powerful scavenger activity for free oxygen radicals, efficiently gathering superoxide anions and hydroxyls.Reference Badary, Taha, Gamal el-Din and Abdel-Wahab18 Thymoquinone is the most active compound of N sativa, and its antioxidant and vasodilator effects have been explicitly observed in recent studies.Reference Tesarova, Svobodova, Kokoska, Marsik, Pribylova and Landa19
Studies so far have demonstrated that the two most important characteristics of thymoquinone are its vasodilatory and scavenger effects. This implies that the two most important pathophysiological mechanisms in the pathogenesis of acoustic trauma, namely free oxygen radical production and reduced blood flow, could be inhibited by thymoquinone. Thus, this study was conducted to determine whether thymoquinone could prevent inner-ear damage caused by acoustic trauma.
Materials and methods
Animals
The study was initiated after approval was obtained from the local ethics board for animal experiments (approval number 2011/05). Thirty-two healthy, mature male Wistar albino rats, weighing 200–240 g, were used in the study. All rats were evaluated otoscopically, and those with pathological findings (including serous otitis, acute otitis and adhesive otitis) were excluded from the study. All rats were housed in an environment with a temperature of 21 ± 1°C, with a 12-hour light/dark cycle, where they had free access to food and water, and where the background noise level was below 50 dB.
Experimental groups
Group 1 (eight rats) was only exposed to acoustic trauma. Group 2 (seven rats) was given thymoquinone 24 hours before the acoustic trauma and continued to receive it for 10 days after the trauma. Group 3 (eight rats) was only treated with thymoquinone, for 10 days. Group 4 (seven rats) was the control group; the rats in this group suffered no trauma and received saline instead of thymoquinone.
Hearing assessment
The pinna reflex test was performed at the beginning of the study for the hearing assessment of all rats. Ketamine 45 mg/kg was injected intramuscularly to induce sedation, after which all rats were examined otoscopically. Any obstructions that might have affected the test results, such as earwax, were removed. Baseline distortion product otoacoustic emission (DPOAE) and auditory brainstem response (ABR) testing was subsequently performed on all rats.
Distortion product otoacoustic emission testing
A GSI Audera™ otoacoustic emission instrument was used for DPOAE measurements. Measurements were carried out in a noise-treated cabin. The DPOAEs were measured using stimulation with different frequencies and intensities. Primary signal levels were adjusted to 65 dB (L1) and 55 dB (L2) for distortion product gram measurements. The two frequencies (f1 and f2) were set as f2/f1 = 1.2. Distortion product gram measurements were carried out at frequencies of 2997, 4002, 5004, 6002, 7001, 8003, 9006, 10005, 11000 and 12000 Hz, as a function of f2. The detection threshold was defined as the primary signal level at which the DPOAE was just distinguishable, at 3 dB above the noise floor. In both measurements, the responses were recorded up to the highest level and the test was concluded.
Auditory brainstem response testing
A Viasys® Medelec Synergy instrument was used for the ABR measurements. Testing was performed on both ears of the anaesthetised rats in a noise-treated cabin. The ABR responses were recorded using needle electrodes placed under the skin. The electrodes were positioned as follows: active electrode in the vertex, ground electrode in the contralateral mastoid and reference electrode in the ipsilateral mastoid. The stimuli were provided through insert earphones using 8 kHz tone-burst sounds with alternating polarities. The filter was adjusted to 30–3000 Hz, the repetition rate was 21 per second and the time window was adjusted to 10 ms. A total of 1024 stimuli were presented for signal averaging.
The test was started by applying stimuli at 80 dB nHL, and the intensity was reduced by 20 dB steps until the threshold value was passed. As we approached the threshold, the intensity was increased by 10 dB each time until the threshold value was reached. At least two traces were performed for each measurement, and attempts were made to repeat the threshold and the threshold was cross-checked. The ABR threshold was defined as the lowest intensity level in which wave III of ABR was observed. Basal ABR measurements carried out prior to the acoustic trauma were compared with ABR measurements on the 1st, 5th and 10th day after the trauma.
Noise exposure
The first two groups of rats were exposed to acoustic trauma using 105 dB SPL white noise for 4 hours. The rats were sedated with an intramuscular injection of ketamine 45 mg/kg, and DPOAE and ABR measurements were carried out on the 1st, 5th and 10th days following acoustic trauma.
Drug administration
Thymoquinone administration began 1 day before exposure to acoustic trauma, and was continued for 10 days after the trauma. Thymoquinone was administered intraperitoneally, at a dose of 10 mg/kg/day.
Sacrification
All experimental animals were sacrificed with 200 mg/kg of intracardiac thiopental using the same method for all animals.
Statistical analysis
This was carried out using the Statistical Package for the Social Sciences version 13.0 for Windows (SPSS, Chicago, Illinois, USA). Data normality was checked using the Kolmogorov–Smirnov test for normality.
The one-way analysis of variance (ANOVA) test was used to compare DPOAE values between groups. The difference between groups was determined with the Bonferroni test (p < 0.05 was accepted as statistically significant). The ANOVA test was also used for the evaluation of measurements taken at different time points among the groups. The difference between time intervals was determined with the Bonferroni post-hoc test (p < 0.05 was accepted as statistically significant).
The Kruskal–Wallis ANOVA test was used to compare ABR thresholds between groups (p < 0.05 was accepted as statistically significant). Differences between groups were investigated using the Mann–Whitney U test. Bonferroni correction was conducted prior to the test (p < 0.0125 was accepted as statistically significant). The Friedman test was used to compare the evaluations among groups (p < 0.05 was accepted as statistically significant). The Wilcoxon signed rank test was used to investigate differences between the groups. Bonferroni correction was performed before the test (p < 0.0125 was accepted as statistically significant).
Results
Distortion product otoacoustic emission test results
Group 1
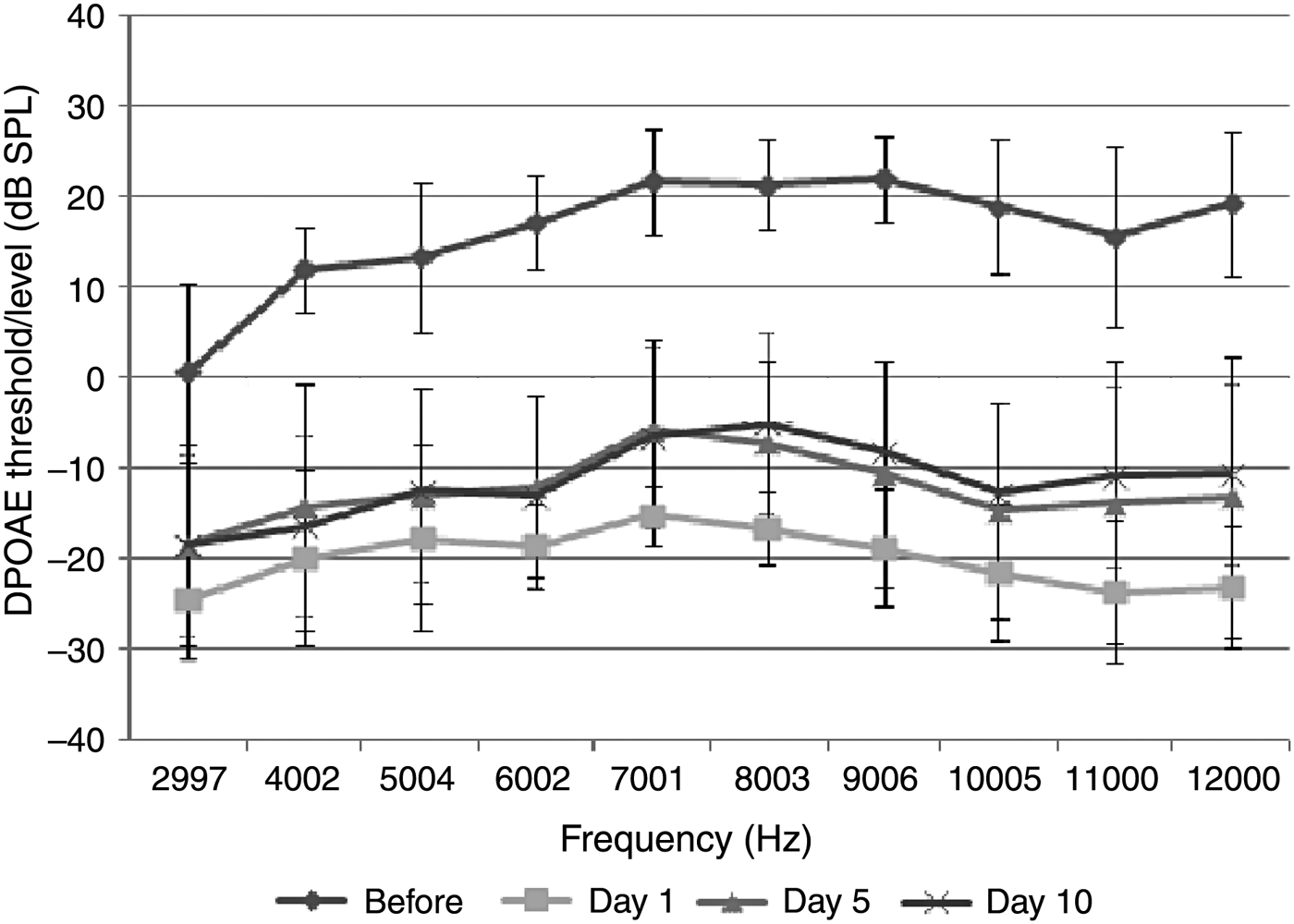
The acoustic trauma group comprised 8 rats (16 ears). Statistically significant differences were observed between distortion product otoacoustic emission (DPOAE) values measured before and on the 1st day after exposure to acoustic trauma (p < 0.05). No significant difference was identified between the DPOAE values measured on the 1st day after acoustic trauma, and those measured on the 5th and 10th days (p > 0.05) (Figure 1). Significant differences were also observed in DPOAE values measured on the 5th and 10th days after exposure to acoustic trauma compared with the baseline measurements (p < 0.05) (Figure 1). These data indicate that the damage caused by acoustic trauma was still present.

Fig. 1 Distortion product grams of group 1 (acoustic trauma group) (error bars represent ± standard deviation). DPOAE = distortion product otoacoustic emissions
Group 2
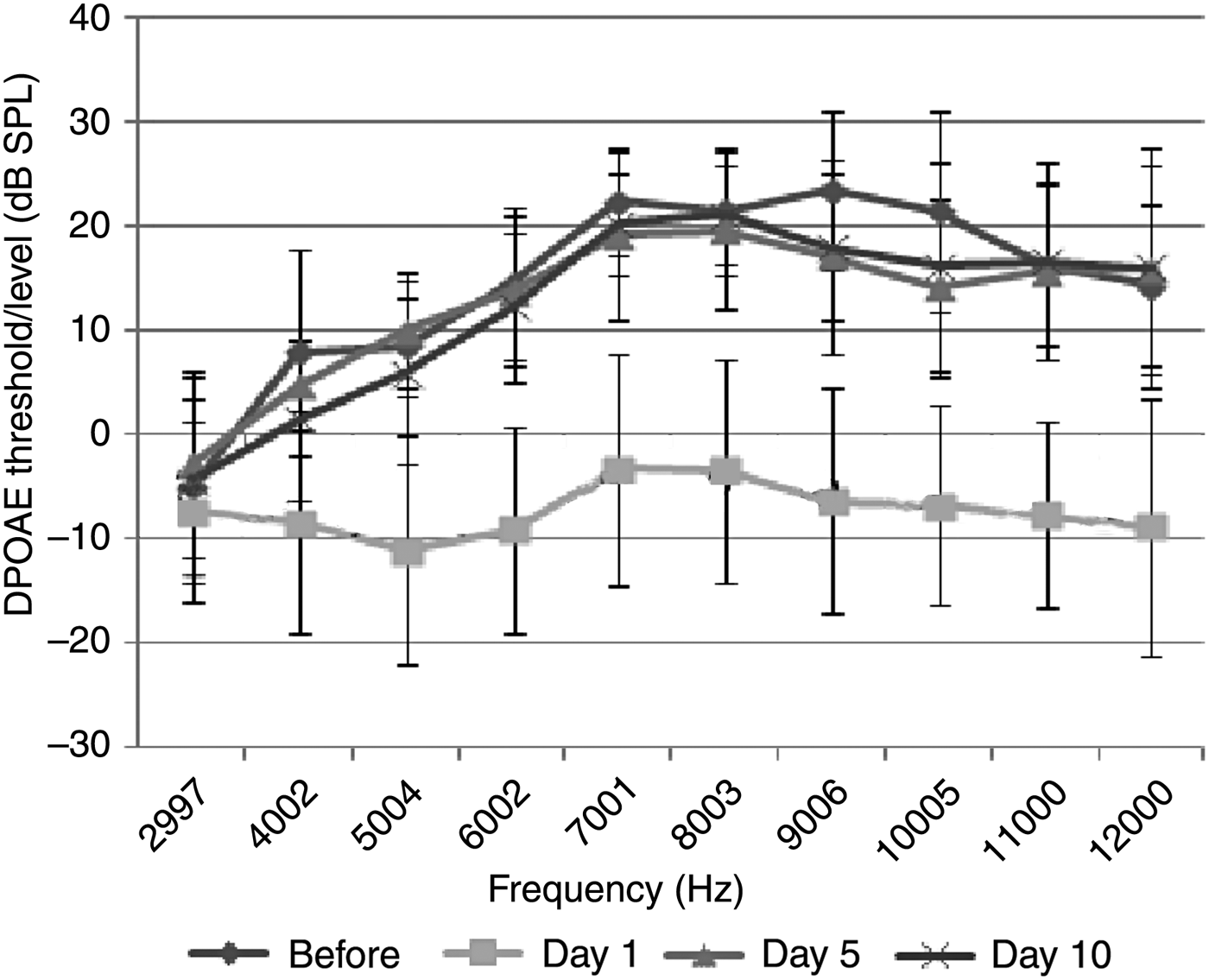
The acoustic trauma plus thymoquinone group comprised 7 rats (14 ears). Statistically significant differences were observed between the DPOAE values measured before and on the 1st day after exposure to acoustic trauma (p < 0.05). This finding demonstrates that acoustic trauma had a significant effect in the cochlea. Significant differences were also observed between the increased DPOAE values measured on the 5th and 10th days after exposure to acoustic trauma compared with the 1st day measurements (p < 0.05). There was no significant difference between the DPOAE values of the 5th and 10th days after acoustic trauma and those before the trauma (p > 0.05). This outcome indicates that thymoquinone has a reparative effect on hearing loss caused by acoustic trauma (Figure 2).

Fig. 2 Distortion product grams of group 2 (acoustic trauma plus thymoquinone group) (error bars represent ± standard deviation). DPOAE = distortion product otoacoustic emissions
Group 3
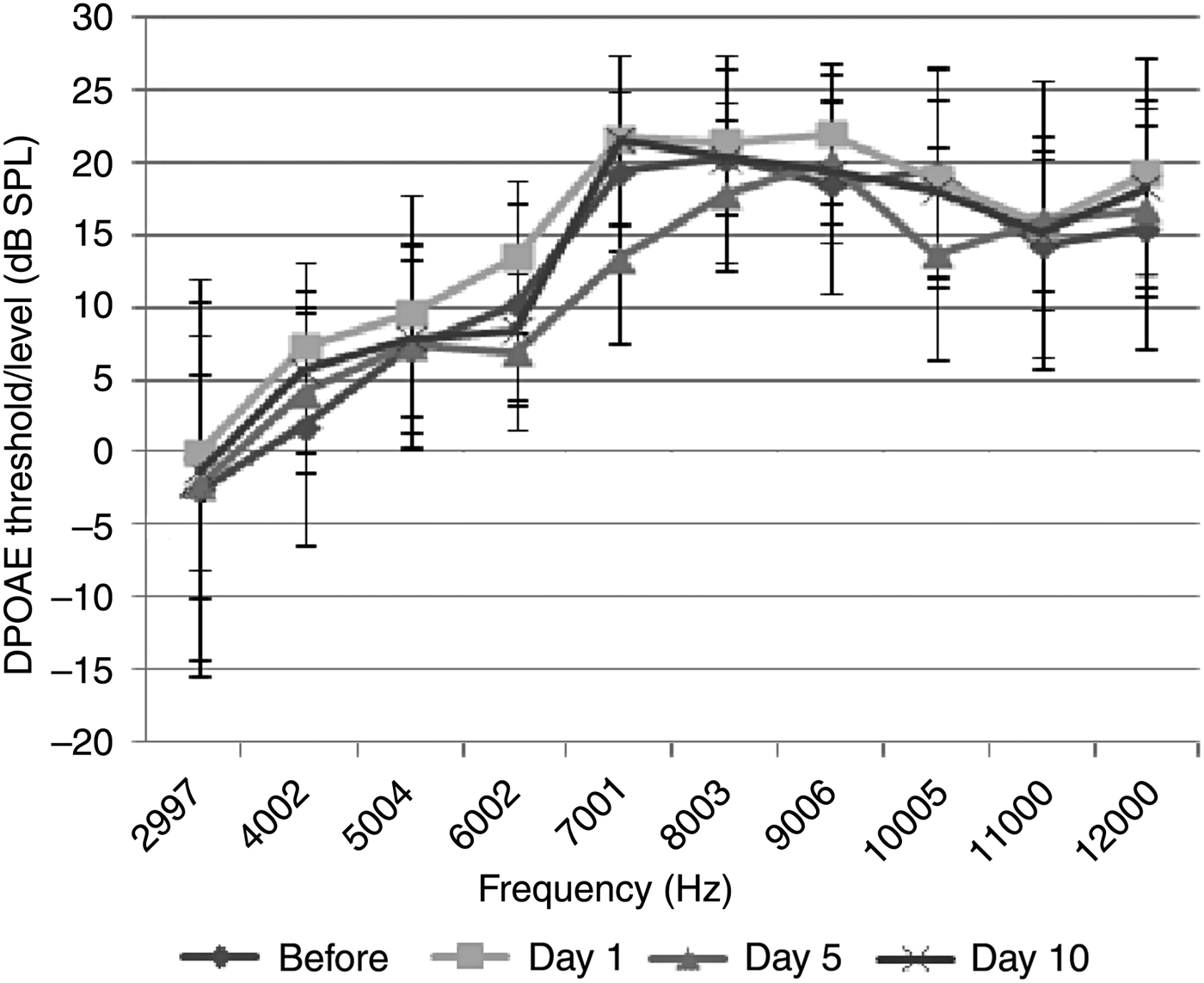
The thymoquinone group comprised 8 rats (16 ears). No statistically significant difference was observed between the baseline measurements and the DPOAE values measured on the 1st, 5th and 10th days (p > 0.05) (Figure 3). Hence, thymoquinone had no detrimental effect on hearing.

Fig. 3 Distortion product grams of group 3 (thymoquinone group) (error bars represent ± standard deviation). DPOAE = distortion product otoacoustic emissions
Group 4
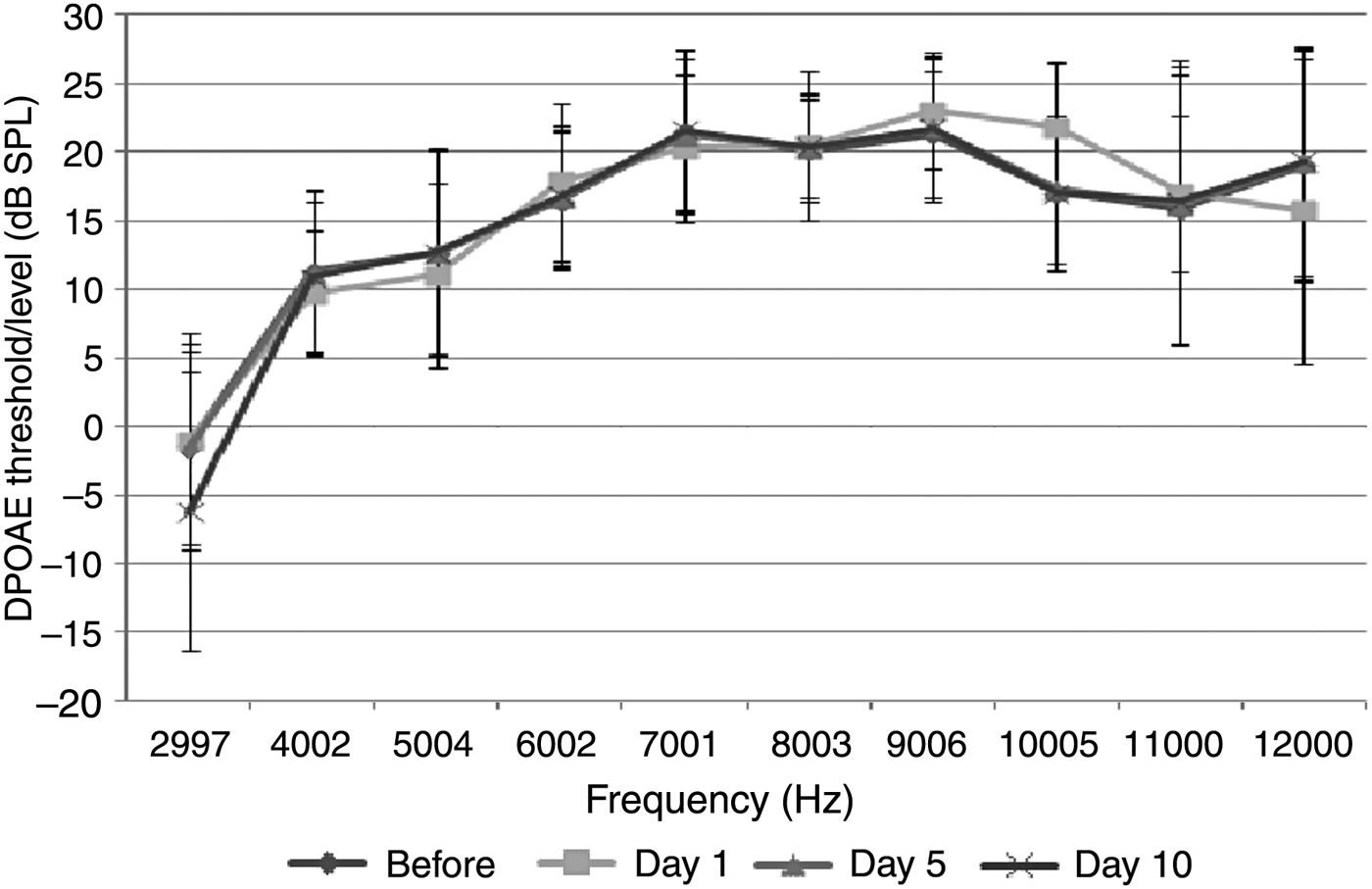
The control group (treated with saline) comprised 7 rats (14 ears). No significant difference was observed between the baseline measurements and the DPOAE values measured on the 1st, 5th and 10th days (p > 0.05) (Figure 4).

Fig. 4 Distortion product grams of group 4 (saline control group) (error bars represent ± standard deviation). DPOAE = distortion product otoacoustic emissions
Auditory brainstem response test results
Group 1
In the acoustic trauma group, the ABR threshold value increased significantly after acoustic trauma exposure (p < 0.0125). No significant difference was identified between the ABR threshold values measured on the 1st day after acoustic trauma and those measured on the 5th and 10th days (p > 0.0125) (Table I).
Table I Auditory brainstem response thresholds

Data represent means ± standard deviations, unless indicated otherwise. *Wilcoxon signed rank test; †Mann–Whitney U test.
Group 2
In the acoustic trauma plus thymoquinone group, the mean ABR threshold increased significantly on the 1st day after acoustic trauma (p < 0.0125). A significant difference was detected between the ABR thresholds on the 1st day after acoustic trauma and thresholds on the 5th and 10th days (p < 0.0125) (Table I).
Group 3
In the thymoquinone group, no significant differences were observed in ABR thresholds between the baseline measurements and measurements taken on the 1st, 5th and 10th days after thymoquinone treatment (p > 0.0125) (Table I).
Group 4
In the control group (treated with saline), no significant differences were observed in ABR thresholds between the baseline measurements and measurements taken on the 1st, 5th and 10th days (p > 0.0125) (Table I).
Group comparison
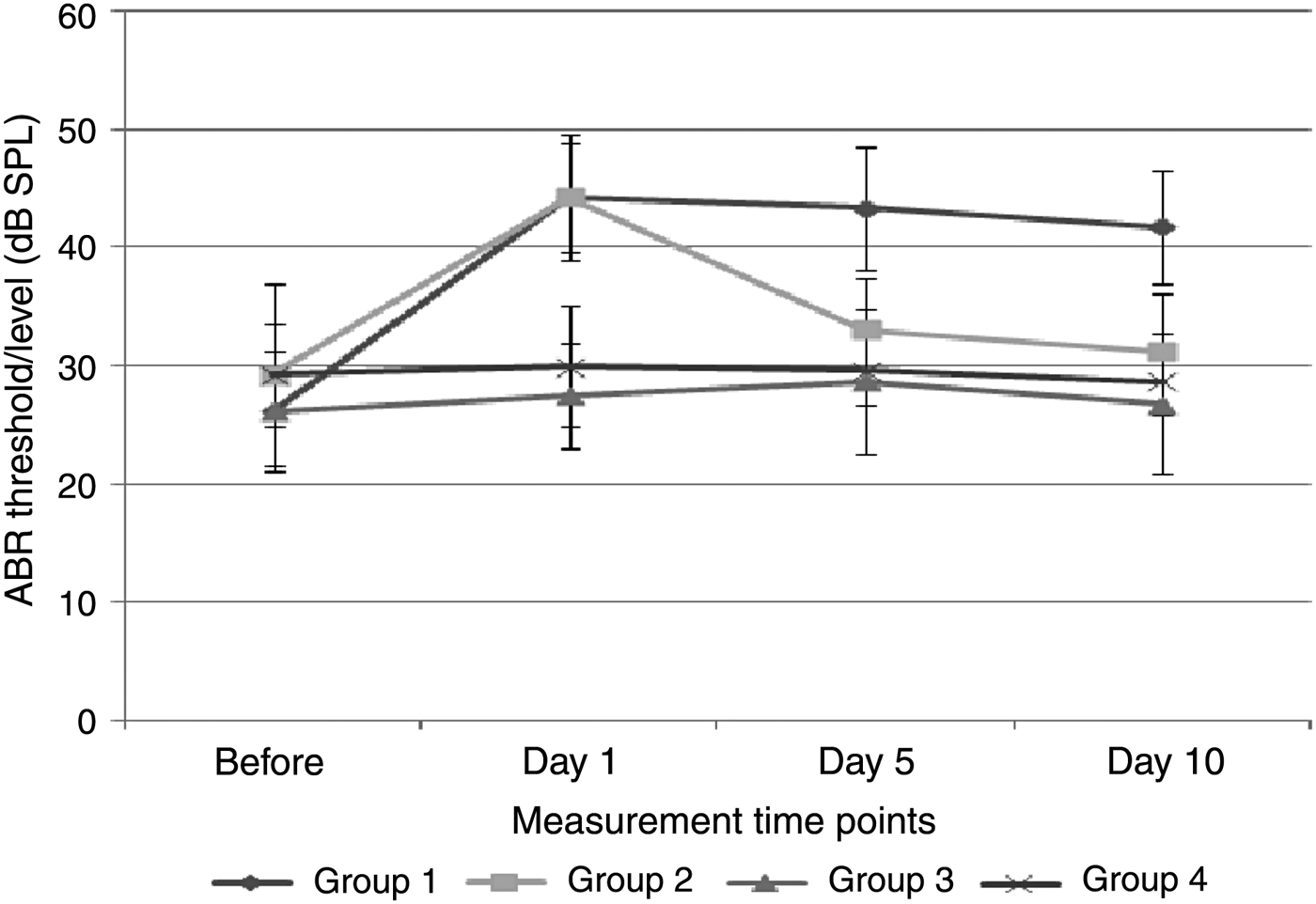
Damage that increased the ABR thresholds was caused by acoustic trauma to the ears of animals in groups 1 and 2. No significant difference was observed between the ABR thresholds of groups 1 and 2 on the 1st day after acoustic trauma (p > 0.0125) (Figure 5). However, a statistically significant difference was detected between the ABR thresholds of groups 1 and 2 measured on the 5th and 10th days after exposure to acoustic trauma (p < 0.0125) (Figure 5). The increase in ABR threshold values remained the same in group 1, while the ABR threshold values reached normal levels in group 2 (following receipt of thymoquinone) (Figure 6). There was no significant difference between the ABR threshold values of group 2 on the 5th and 10th days after the trauma and the threshold values before trauma (p > 0.05).

Fig. 5 Comparison of auditory brainstem response (ABR) thresholds among the groups.

Fig. 6 Example graphs of auditory brainstem response thresholds before acoustic trauma (a), after acoustic trauma (b), and after acoustic trauma and thymoquinone administration (c).
There were statistically significant differences between groups 1 and 3, and between groups 1 and 4, in terms of ABR thresholds on the 1st, 5th and 10th days (p < 0.0125) (Figure 5). There were no statistically significant differences between groups 3 and 4 on the 1st, 5th and 10th days (p > 0.0125) (Figure 5).
Discussion
The pathogenesis of acoustic trauma is multifactorial. It may lead to the loss of hair cells, disorders of stereocilia, collapse of support cells, rupture of dendrites, and to various other structural and metabolic damage such as strial oedema.Reference Wang, Hirose and Liberman20 Thymoquinone is an antioxidant with strong scavenger properties; it also has a vasodilatory effect, which enhances blood flow. This study aimed to investigate the preventive effect of thymoquinone on hearing loss caused by acoustic trauma, using distortion product otoacoustic emission (DPOAE) and ABR methods.
Broadband noise (white noise), with constant amplitude over the whole frequency spectrum, causes homogeneous damage throughout the cochlea.Reference Bogoyevitch, Boehm, Oakley, Ketterman and Barr21 In this study, 105 dB broadband noise was applied to two groups of rats (group 1 (acoustic trauma group) and group 2 (acoustic trauma plus thymoquinone group)) for 4 hours to induce acoustic trauma. The ABR and DPOAE measurements were carried out on the 1st day after trauma in these groups; when compared with baseline measurements, these values showed a statistically significant difference, which confirmed that acoustic trauma was successful in inducing hearing loss. No significant difference was observed in ABR thresholds between groups 1 and 2 on the 1st day following acoustic trauma (Table I), which indicated that thymoquinone treatment initiated 24 hours before acoustic insult did not have a preventive effect on acoustic trauma.
A significant difference was observed between ABR threshold values in groups 1 and 2 measured on the 5th and 10th days after exposure to acoustic trauma (Figure 5). The DPOAE and ABR values on the 5th and 10th days in group 2 were restored to baseline values with thymoquinone treatment, and the effects of acoustic trauma were completely eliminated and relieved. These findings show that thymoquinone helps to repair damage to the inner ear caused by acoustic trauma. In group 3 (thymoquinone group), there were no significant differences in either ABR thresholds or DPOAE values on the 1st, 5th and 10th days after the trauma compared with baseline measurements (Figures 3 and 5). This suggests that thymoquinone has no negative effect on hearing. This is the first study in the medical literature to investigate the relationship between thymoquinone and acoustic trauma, and the first to show the rapid reparative effect of thymoquinone on the damage caused by acoustic trauma.
Apoptosis and necrosis of hair cells in the cochlea are important factors in hearing loss induced by acoustic trauma.Reference Hu, Henderson, Nicotera, Simmons and Palmer22 Apoptosis begins on the 1st day following exposure to noise.Reference Hu, Henderson and Nicotera23 The increase in ABR threshold values and the decrease in DPOAE values in groups 1 and 2 after acoustic trauma may be the consequence of apoptosis. After the initial stage, necrosis and apoptosis contribute equally to sensory cell loss.Reference Yang, Henderson, Hu and Nicotera24 Thymoquinone may prevent apoptosis by inhibiting Bcl-2 related peptide and auditory brainstem response derivatives.Reference Gali-Muhtasib, Diab-Assaf, Boltze, Al-Hmaira, Hartiq and Roessner25 In our study, DPOAE values returned to normal levels on the 5th day in group 2 animals that were traumatised and treated with thymoquinone. From this outcome, we believe that thymoquinone has a positive effect on repairing cells undergoing apoptosis and on preventing necrosis.
It was recently reported that thymoquinone increases blood flow in most tissues by increasing metabolism, and, as a result, more oxygen is directed towards cells affected by stress.Reference Sankaranarayanan and Pari26 Exposure to loud noise decreases blood flow in the cochlea.Reference Miller, Yamashita, Minami, Yamasoba and Le Prell5 The reduction of blood flow in noise-induced hearing loss induces ischaemia and results in the increased production of free oxygen radicals. Antioxidants used in noise-induced hearing loss reduce free oxygen radical formation leading to vasoconstriction. Thymoquinone may treat cochlear damage by reducing the amount of oxygen radicals and by increasing blood flow (via its vasodilatory effect). In our study, the decrease in DPOAE values after trauma was improved with thymoquinone administration. This indicates that thymoquinone has a reparative effect on outer hair cells.
Acoustic trauma creates acoustic energy in the cochlea, and free oxygen radicals are produced as a result of transformation of this energy into neural signals. Endogenous protective mechanisms exist to prevent free oxygen radical production in the cochlea. These mechanisms are the antioxidant systems, traffic factors and heat shock proteins.Reference Shoji, Miller, Mitchell, Yamasoba, Altschuller and Miller27 Strengthening natural antioxidant mechanisms prevents or reduces hearing loss.Reference Kopke, Weisskopf, Boone, Jackson, Wester and Hoffer28
H1-receptor antagonists, corticosteroids, vasodilators, anticoagulants, volume expanders, antioxidant agents such as vitamin A, C and E, and many other agents such as magnesium and hyperbaric oxygen, have been employed in the treatment of acoustic trauma, either by themselves or in various combinations.Reference Le Prell, Hughes and Miller29 In this study, the benefits of thymoquinone were observed in as short a time as 5 days. This is likely to be the result of thymoquinone activating the endogenous antioxidant system, and of the antioxidant and vasodilatory effects of thymoquinone itself.Reference Tesarova, Svobodova, Kokoska, Marsik, Pribylova and Landa19
The use of antioxidants is the most important action in treating acoustic trauma. Because free oxygen radical production plays an important role in the pathogenesis of many neurodegenerative syndromes, antioxidant agents have a therapeutic effect in neurodegenerative processes.Reference Wang, Hirose and Liberman20 Various animal studies have shown that exogenous antioxidant agents reduce sensorial cell death and noise-induced hearing loss, and increase the effects of free radical collectors.Reference Duan, Qiu, Laurell, Olofsson, Counter and Borq30 It has also been demonstrated that thymoquinone reacts with glutathione, creating a very powerful free radical collector referred to as glutathionyl-thymohydroquinone.Reference Khalife and Lupidi31 Thymoquinone is a direct antioxidant agent and it stimulates the endogenous antioxidant system. The return of DPOAE values and ABR thresholds to normal levels in just 5 days in the thymoquinone-treated group (group 2) might be the result of thymoquinone's strong antioxidant effect.
In this study, thymoquinone administration was started 1 day before acoustic trauma and was continued for 10 days. It was administered intraperitoneally at a dose of 10 mg/kg/day. In the literature, the optimal administration time for antioxidants to induce effective protection against acoustic trauma varies. Some studies indicate that the reparative constituent must be used before acoustic trauma,Reference Henderson, Bielefeld, Harris and Hu12 while others indicate that it should be used immediately after, or for 5 days after exposure to noise, to reduce late-term free radical formation.Reference Yamane, Nakai, Takayama, Iqucgi, Nakaqawa and Kojima11 For this reason, thymoquinone treatment should not need to last for more than 10 days. Similar measurements of DPOAE and ABR taken on the 5th and 10th days in this study confirm that it is unnecessary to administer thymoquinone for more than 10 days.
• The pathogenesis of acoustic trauma is multifactorial
• This study aimed to investigate the preventive effect of thymoquinone on hearing loss caused by acoustic trauma
• Thymoquinone is a direct antioxidant agent and it stimulates the endogenous antioxidant system
• This is the first study to show the rapid reparative effect of thymoquinone on damage caused by acoustic trauma
This is the first study in the medical literature to investigate the relationship between thymoquinone and acoustic trauma in rats, and the first to show the rapid reparative effect of thymoquinone on damage caused by acoustic trauma. This is due to its dual effect: by itself, thymoquinone has strong antioxidant and vasodilator properties, while it also activates the endogenous antioxidant system. Further studies are needed to demonstrate its therapeutic effects in humans.