Introduction
Chronic rhinosinusitis causes respiratory and olfactory dysfunction and results in symptoms such as nasal blockage, rhinorrhoea, facial pain or pressure, headache, loss of smell, sleep disturbance or fatigue, sputum, and cough.Reference Fokkens, Lund, Hopkins, Hellings, Kern and Reitsma1 The prevalence of symptom-based chronic rhinosinusitis in the population has been reported to be 5.5–28 per cent.Reference Fokkens, Lund, Hopkins, Hellings, Kern and Reitsma1
Eosinophilic chronic rhinosinusitis has been thought to be an intractable, diffusely bilateral chronic rhinosinusitis since it was reported in 2001.Reference Fujieda, Imoto, Kato, Ninomiya, Tokunaga and Tsutsumiuchi2,Reference Haruna, Otori, Yanagi and Moriyama3 The number of eosinophilic chronic rhinosinusitis patients has recently been reported to be increasing significantly. Eosinophilic chronic rhinosinusitis is clinically characterised by: bilateral chronic rhinosinusitis with nasal polyposis, olfactory loss in the early stage, late-onset asthma, peripheral blood eosinophilia, severe eosinophilic infiltration of the sinonasal mucosae, lesions predominantly involving the ethmoid sinuses and olfactory clefts on computed tomography (CT), refractory to macrolide therapy, response to corticosteroids (steroids), and frequent recurrence of nasal polyposis. The diagnostic criteria are based on a multicentre study of the Japanese Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis.Reference Tokunaga, Sakashita, Haruna, Asaka, Takeno and Ikeda4 Eosinophilic chronic rhinosinusitis has been treated as an incurable disease as designated by the Ministry of Health, Labour and Welfare in Japan since 2015. Chronic rhinosinusitis not only significantly worsens patients’ quality of life, but it also has adverse health and economic effects.Reference Rudmik5
To obtain sufficient therapeutic effects in eosinophilic chronic rhinosinusitis, appropriate management by well combined medical and surgical treatments based on the aetiology and pathogenesis of eosinophilic chronic rhinosinusitis is required. Inflammatory endotypes based on a pathophysiological mechanism have been studied.Reference Tomassen, Vandeplas and Van Zele6 Functional endoscopic sinus surgery (FESS) is the ‘gold standard’ procedure when the disease is refractory to prior medical treatment using antibiotics, mucolytics, anti-allergic medicines and steroids. Full-house FESS, indicating opening of all sinuses without any residual cells, is recommended.Reference Okushi, Mori, Nakayama, Asaka, Matsuwaki and Ota7 FESS offers the advantages of being able to remove lesions, irrigate and quickly improve symptoms. For post-operative treatment, FESS is also beneficial for topical drug delivery. However, sinonasal polyp recurrence for up to 18 months after FESS has still been reported as common, occurring in 60–70 per cent of chronic rhinosinusitis patients.Reference DeConde, Mace, Levy, Rudmik, Alt and Smith8 At the present time, long-term prevention of recurrence after FESS is an important task in the management of eosinophilic chronic rhinosinusitis. Investigation into both surgical and medical management strategies is warranted to improve the observed prevalence of recurrence.
Steroids are used to reduce inflammation and inhibit the immune system.Reference Grennan and Wang9 Steroid therapy is undeniably essential in the medical treatment of chronic rhinosinusitis.Reference Fokkens, Lund, Hopkins, Hellings, Kern and Reitsma1 The beneficial effects of both topical and systemic steroid treatments to relieve the symptoms due to chronic rhinosinusitis have been reported with high evidence levels.Reference Fokkens, Lund, Hopkins, Hellings, Kern and Reitsma1,Reference Miwa, Furuta, Shiga, Ikeda, Kondo and Tsuzuki10 When using steroids while trying to avoid adverse reactions, the issue of adrenal function suppression should be considered in cases of systemic administration.Reference Grennan and Wang9 In several topical therapy strategies that were evaluated based on the previous evidence, topical sinonasal steroid therapies were recommended as an option for managing chronic rhinosinusitis in addition to sinonasal saline irrigation.Reference Rudmik, Hoy, Schlosser, Harvey, Welch and Lund11,Reference Bardaranfar, Ranjbar, Dadgarnia, Atighechi, Mirvakili and Behniafardet12 Thus, use of a protocol for post-FESS therapy that incorporated topical steroid treatment using triamcinolone acetonide with oxidised regenerated cellulose has been reported to reduce the post-operative amount of oral steroid without adrenal suppression in eosinophilic chronic rhinosinusitis patients.Reference Tokunaga, Sakashita, Haruna, Asaka, Takeno and Ikeda4
The purpose of this study was to: (1) clarify the indications for sinonasal topical steroid treatments for eosinophilic chronic rhinosinusitis patients and (2) evaluate the therapeutic effects of sinonasal topical steroid treatments in post-operative eosinophilic chronic rhinosinusitis patients. We also examine the current positioning of steroid therapy in the treatment strategy for eosinophilic chronic rhinosinusitis when biologic drugs are indicated for eosinophilic chronic rhinosinusitis with nasal polyps.
Materials and methods
Patients
Between January 2016 and February 2020, 30 adult patients (22 men and 8 women; median age, 48 years; age range, 28–75 years) with eosinophilic chronic rhinosinusitis who underwent bilateral primary FESS were retrospectively enrolled in this study. Full-house FESS was performed by three rhinologists using a navigation system (Fusion ENT navigation system, Jacksonville, USA) under general anaesthesia for all patients.
Chronic rhinosinusitis was diagnosed by clinical symptoms persisting for three months or more, sinonasal examination by endoscopy and CT.Reference Fokkens, Lund, Hopkins, Hellings, Kern and Reitsma1 Eosinophilic chronic rhinosinusitis was diagnosed when the total score of the following 4 items was 11 or more: (1) bilateral lesions (3 points); (2) nasal polyps (2 points); (3) dominant ethmoid sinus involvement or pansinusitis on CT (2 points); and (4) blood eosinophils more than 2 per cent but equal to or less than 5 per cent (4 points), more than 5 per cent but equal to or less than 10 per cent (8 points), and more than 10 per cent (10 points), according to the criteria based on the Japanese Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis Study study.Reference Tokunaga, Sakashita, Haruna, Asaka, Takeno and Ikeda4
Patients not fulfilling the eosinophilic chronic rhinosinusitis diagnostic criteria and those with tumour-associated diseases, trauma and history of any previous sinonasal surgery were excluded from this study.
This study used a case series design and conformed to the regulations of the ethics committee of Hyogo College of Medicine (approval numbers: 1512 and 3308). This study was performed in accordance with the principles of the Declaration of Helsinki.
Sinonasal topical steroid treatment
As post-operative treatment, a sinonasal topical steroid treatment using a bioabsorbable device consisting of oxidised regenerated cellulose (Surgicel absorbable haemostat, Johnson and Johnson, Tokyo, Japan) and triamcinolone acetonide (Kenacort, 40 mg/ml vial, Bristol Myers Squibb, Tokyo, Japan) was performed based on a previous report.Reference Konno, Kashiwagi, Tsunemi, Goto and Haruna13
First, several cotton swabs impregnated with 4 per cent xylocaine and 5000-fold diluted adrenaline were bilaterally inserted into the surgically opened ethmoid sinuses and the olfactory cleft regions to shrink the nasal mucosae for 5–10 minutes. Second, a moderate amount of oxidised regenerated cellulose was inserted into the bilateral ethmoid sinuses and olfactory clefts. Third, triamcinolone acetonide with a half vial of triamcinolone acetonide (20 mg/0.5 ml) was dripped onto the oxidised regenerated cellulose on each side.
We investigated two groups: group A with patients who underwent sinonasal topical steroid treatment after FESS, and group B with control patients who did not undergo sinonasal topical steroid treatment after FESS. Oral steroids were not administered to any patients during the study period.
Nasal symptoms questionnaire
The nasal symptoms questionnaire is a self-administered questionnaire consisting of 10 items with 2 parts. Part one included eight nasal symptom-related items of: (1) sneezing or itching of the nose, (2) nasal discharge, (3) nasal obstruction, (4) post-nasal drip or sputum, (5) olfactory loss, (6) pain (tooth, buccal, facial pain or headache), (7) eye itching or epiphora, and (8) cough or feeling of irritation in the throat. Part two included two quality of life related items of: (9) reduced productivity at school or work, limitation of outdoor life or social functioning, and (10) sleep, general physical or emotional problems (Figure 1).Reference Saito, Tsuzuki, Nishikawa, Okazaki, Hashimoto and Sakagami14

Fig. 1. Nasal symptoms questionnaire.
The severity of each nasal symptoms questionnaire component was divided into four levels: 0 points (no symptoms at all); 1 point (mild); 2 points (severe); or 3 points (extremely severe).
The total nasal symptoms questionnaire score (ranging from 0 to 30 points) and its components were analysed. In the third part, a visual analogue scale for nasal symptoms that consisted of a 10-cm linear scale, with the opposing ends as the normal (0 per cent) and most severe (100 per cent) extremes, was also administered.
Post-operative endoscopic appearance score
The post-operative endoscopic appearance of the operated sinuses and olfactory clefts was scored as follows: 0 (normal); 1 (partially observable due to presence of polyps, oedematous mucosa or discharge); and 2 points (unobservable due to being completely filled with swollen mucosae, polyps or discharge).Reference Tsuzuki, Hinohira, Takebayashi, Kojima, Yukitatsu and Daimon15 When the polyps occupied and prevented observation of the posterior part of the sinuses, a score of 2 points was assigned to the posterior part of the sinuses that had been operated on. Sinuses that had not been operated on were excluded from scoring. The percentage of the total score relative to the maximum possible worst score for operated sinuses was rated as the post-operative endoscopic appearance score.
Computed tomography score
In order to evaluate the degree of severity of chronic rhinosinusitis, sinonasal CT findings were scored based on the Lund and Mackay scoring system.Reference Lund and Mackay16 The frontal, maxillary, anterior and posterior ethmoid and sphenoid sinuses were scored as 0 (no opacification), 1 (partial opacification) or 2 points (complete opacification). The ostiomeatal complex was scored as 0 (not opaque) or 2 points (with opacification). The CT score was the sum of the scores at each site. The CT score ranged from 0 to 12 points per side (bilateral range, 0–24 points).
Olfactory evaluation
In order to evaluate olfactory acuity, the T&T olfactometer recognition threshold test (Daiichi Yakuhin Sangyo, Tokyo, Japan), which is covered by health insurance in Japan, was performed as reported previously.Reference Oka, Tsuzuki, Takebayashi, Kojima, Daimon and Sakagami17 The T&T olfactometer consists of five odorants: (1) β-phenyl ethyl alcohol, which smells like a rose; (2) methyl cyclopentenolone, which smells like burnt caramel; (3) iso-valeric acid, which smells like sweat; (4) γ-undecalactone, which smells like peach; and (5) skatole, which smells like garbage. Recognition thresholds for each odorant were obtained, averaged and evaluated as the mean olfactory recognition threshold.
Statistical analysis
Comparisons of results between groups A and B were analysed using the Mann–Whitney U test. Differences in scores over time between before (day 0) and after sinonasal topical steroid treatment (days 7–28) in group A were analysed using the Wilcoxon signed rank-sum test. Data are presented as median values (range), unless otherwise indicated. All p-values are two-sided, and values of p < 0.05 were considered significant. All statistical analyses were performed using Stat Flex (version 6.0) statistical software (Osaka, Japan).
Results
Comparison of groups
To clarify the indications for sinonasal topical steroid treatment for post-operative eosinophilic chronic rhinosinusitis patients, differences in the clinical characteristics in the pre- and post-operative stages between groups A (just before sinonasal topical steroid treatment at day 0, n = 15) and B (worst scores, n = 15) were investigated.
Group A was significantly younger than group B (p = 0.0084), and the pre-operative CT score was significantly higher in group A than in group B (p = 0.0208). Non-significant differences in sex, presence of bronchial asthma, Japanese Epidemiological Survey of Refractory Eosinophilic Chronic Rhinosinusitis score, total nasal symptoms questionnaire score, visual analogue scale, mean olfactory recognition threshold, and total immunoglobulin E level between groups A and B were observed (Table 1).
Table 1. Baseline characteristics of patients with eosinophilic chronic rhinosinusitis in the pre-operative stage by group

*n = 15; †n = 15; ‡p < 0.01; **p < 0.05. P-values indicate differences between groups A and B
In the post-operative stage, total nasal symptoms questionnaire scores showed significant improvement in symptoms in both groups A (p < 0.001) and B (p < 0.01) compared with their scores before FESS. Olfactory loss, a nasal symptoms questionnaire component (median value, 3 points) in group A was found to be significantly more severe even than that of the worst score in group B (median value, 1 point; p = 0.0289). The post-operative endoscopic appearance score was significantly worse in group A (median value, 50.0 per cent; range, 0–66.7 per cent) than in group B (median value, 12.5 per cent; range, 0–83.3 per cent; p = 0.0019; Figure 2). All components of the post-operative endoscopic appearance score were significantly higher in group A than in group B, except in the maxillary sinus.

Fig. 2. Sinonasal conditions of groups A and B in the post-operative stage. Graph shows data in group A just before sinonasal topical steroid treatment at day 0 (n = 15) and group B, with the worst conditions compared and analysed (upper lane). Differences in each site between the two groups are shown in the lower lane. Asterisks indicate significant differences between groups A and B using the Mann–Whitney U test (*p < 0.05, **p < 0.01). PEAS = post-operative endoscopic appearance score
Effects of sinonasal topical steroid treatment
In order to evaluate therapeutic effects, changes in the nasal symptoms questionnaire between before (day 0) and after sinonasal topical steroid treatment (days 7, 14, 21 and 28) were investigated in group A. Total nasal symptoms questionnaire scores were significantly improved and maintained for four weeks during the target period in this study, although the nasal symptoms questionnaire scores tended to increase over time (Figure 3a).

Fig. 3. Changes in symptoms after sinonasal topical steroid treatment. (a) Total nasal symptoms questionnaire score and (b) visual analogue scale score at each time period. Asterisks indicate significant differences between before and after local steroid treatment using the Wilcoxon signed-rank test (*p < 0.05, **p < 0.01).
Significant improvements of nasal symptoms questionnaire components were found for three symptoms (nasal obstruction, nasal discharge and olfactory loss; all p < 0.05). Improvement rates for nasal obstruction were 53.3 per cent (day 7), 33.3 per cent (days 14 and 21) and 30.8 per cent (day 28). Nasal discharge and olfactory loss showed higher improvement rates of 66.7 per cent (day 7), 60.0 per cent (day 14 and 21) and 38.5 per cent (day 28), and 60.0 per cent (day 7), 66.7 per cent (day 14), 53.3 per cent (day 21) and 38.5 per cent (day 28), respectively. Similar to the total nasal symptoms questionnaire score, the visual analogue scale score also tended to become worse over time, but a significant improvement was maintained (Figure 3b).
Non-significant differences in the post-operative endoscopic appearance score between before (median value, 50.0 per cent; range, 29.2–66.7 per cent on day 0) and after (median value, 50.0 per cent; range, 0–66.7 per cent on day 28) treatment were found.
No patients showed any symptoms of hypoadrenocorticism. The blood levels of adrenocorticotropic hormone were maintained within the normal range (normal range, 7.2–63.3 pg/ml) in all patients. Although 4 patients had low blood levels of cortisol in the range from 3.09 to 5.95 pg/ml (normal range, 6.24–18.0 pg/ml), their blood cortisol levels were normalised without any steroids for 2–4 months.
Discussion
This study clarified the indications and effects of sinonasal topical steroid treatment in post-operative patients with eosinophilic chronic rhinosinusitis.
Younger age is reported to be one of the exacerbating factors of eosinophilic chronic rhinosinusitis in adult patients.Reference Tsuzuki, Hashimoto, Okazaki, Nishikawa and Sakagami18 Patients with a higher pre-operative CT score, indicating more severe inflammation, showed a more severe olfactory disorder before FESS.Reference Saito, Tsuzuki, Yukitatsu and Sakagami19 A higher pre-operative CT score also showed more severe operative findings and a higher post-operative endoscopic appearance score, indicating relapsing sinonasal polyps after FESS, in patients with eosinophilic chronic rhinosinusitis.Reference Tsuzuki, Hashimoto, Okazaki and Sakagami20 The present study also suggests that patients with olfactory loss after FESS require more robust post-operative treatment. Based on the results of the present study, it is thought that younger age, higher pre-operative CT score, severe post-operative olfactory loss and worse sinonasal conditions lead to the need for sinonasal topical steroid treatment.
Regarding the methodology of sinonasal topical steroid treatment, triamcinolone acetonide is mainly administered to the bilateral surgically opened ethmoid sinuses and olfactory clefts. The posterior ethmoid and the olfactory cleft are thought to be initially involved in eosinophilic chronic rhinosinusitis.Reference Ishitoya, Sakuma and Tsukuda21,Reference Sakuma, Ishitoya, Komatsu, Shiono, Hirama and Yamashita22 Sinonasal conditions in eosinophilic chronic rhinosinusitis tend to recur earlier from the frontal sinus pathway after FESS.Reference Tsuzuki, Hashimoto, Okazaki, Nishikawa and Sakagami18 Sinonasal conditions were significantly worse in group A than in group B in the present study. Topical treatment that can deliver steroids directly to these lesions is rational and beneficial in the post-operative management of eosinophilic chronic rhinosinusitis. Considering the adverse effects of steroids, topical rather than systemic therapy is preferable and prescribed whenever possible.Reference Grennan and Wang9 However, locally applied steroids are sometimes absorbed systemically to cause adverse effects. With the topical steroid treatment in the present study, patients who showed decreasing cortisol levels recovered after 2–4 months without any manifestations considered to indicate adrenal suppression. Thus, sinonasal topical steroid treatment appears to be one of the safe treatments when monitoring the patient's physical condition and blood levels of cortisol, as reported previously.Reference Konno, Kashiwagi, Tsunemi, Goto and Haruna13
In order to investigate therapeutic effects on symptoms in patients with eosinophilic chronic rhinosinusitis, we used a scoring system known as the nasal symptoms questionnaire that we previously proposed.Reference Saito, Tsuzuki, Nishikawa, Okazaki, Hashimoto and Sakagami14 The nasal symptoms questionnaire score has been verified to have a high area under the receiver operating characteristic curve, internal consistency and test–retest reproducibility. According to the nasal symptoms questionnaire score results in the present study, significant improvements of the nasal symptoms questionnaire scores after FESS in both groups showed that FESS is a useful treatment for relieving the symptoms of eosinophilic chronic rhinosinusitis patients. In post-operative management, sinonasal topical steroid treatment could also maintain significant improvement of total nasal symptoms questionnaire scores for four weeks. Of the nasal symptoms questionnaire components, significant effects on nasal discharge, nasal obstruction and olfactory loss were observed, whereas there were no effects on pain, eye and lower respiratory symptoms. In particular, the data that showed that olfactory loss continued to show significant improvement until three weeks confirmed the results of the previous study.Reference Konno, Kashiwagi, Tsunemi, Goto and Haruna13
The efficacy of both topical and systematic steroid administration for nasal symptoms has been reported from randomised, double-blind studies with high evidence levels.Reference Miwa, Furuta, Shiga, Ikeda, Kondo and Tsuzuki10,Reference Ikeda, Sakurada, Suzaki and Takasaka23,Reference Stjärne, Mösges, Jorissen, Passàli, Bellussi and Staudinger24 Steroids recover the barrier function of adherens and tight junctions weakened in the endoepithelial cells of inflammatory sinonasal mucosae.Reference Yukitatsu, Hata, Yamanegi, Yamada, Ohyama and Nakasho25 Consequently, steroids are thought to be effective therapeutic agents for conductive olfactory disorders to reduce the volume of nasal polyps through anti-oedema effects.
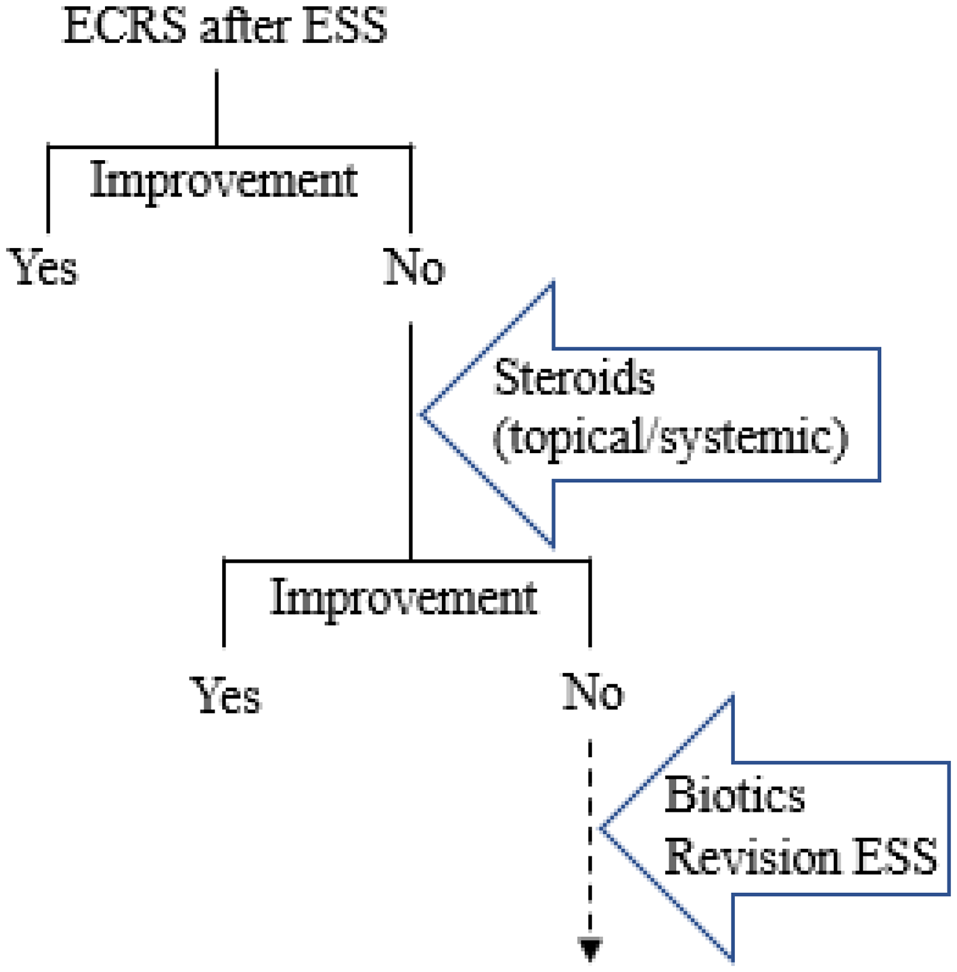
The strategy for management of intractable eosinophilic chronic rhinosinusitis after primary FESS is suggested in Figure 4. Post-operative treatments are also of importance to maintain the improvement of symptoms of eosinophilic chronic rhinosinusitis patients. In particular, medical treatments are continuously required in eosinophilic chronic rhinosinusitis patients to prevent recurrence after FESS. Steroids, which are among the most effective anti-inflammatory medications for allergic diseases, are topically or systemically administered while monitoring for adverse effects.Reference Barnes26 The dosing and duration of steroid treatment for eosinophilic chronic rhinosinusitis are still open issues at present.
• Numbers of eosinophilic chronic rhinosinusitis patients have recently been reported to be increasing significantly
• This study aimed to clarify indications for sinonasal topical steroid treatments for post-operative eosinophilic chronic rhinosinusitis patients
• Steroid treatment is needed with younger age, higher pre-operative computed tomography score, severe olfactory loss and worse sinonasal conditions
• Total nasal symptom scores were significantly improved and maintained for four weeks during this study
• Sinonasal topical steroid treatment appears to be a beneficial post-operative therapy for patients with eosinophilic chronic rhinosinusitis

Fig. 4. Strategy for post-operative management of eosinophilic chronic rhinosinusitis after primary functional endoscopic sinus surgery. Improvement should be comprehensively determined by patients’ subjective symptoms (questionnaire), nasal function tests (olfaction test, rhinomanometry), blood examination (eosinophilia) and sinonasal conditions by endoscopy or imaging (computed tomography). ECRS = eosinophilic chronic rhinosinusitis; ESS = endoscopic sinus surgery
For topical steroid treatments, a method of exhalation through the nose of inhaled steroids, a retronasal delivery system, has been reported to be useful for patients with eosinophilic chronic rhinosinusitis and comorbid asthma as an airway medicine that simultaneously treats inflammation of both the upper and lower respiratory tract.Reference Kobayashi, Yasuba, Asako, Yamamoto, Takano and Tomoda27,Reference Kanda, Kobayashi, Asako, Tomoda, Kawauchi and Iwai28 The therapeutic effects of sinonasal topical steroid treatment were also demonstrated in the present study. When the patient's symptoms are resistant to post-operative steroid treatment,Reference Adcock and Barnes29 further revision surgery to remove the inflamed lesions and residual cellsReference Okushi, Mori, Nakayama, Asaka, Matsuwaki and Ota7 or biological therapies without serious adverse events, such as dupilumab,Reference Bachert, Mannent, Naclerio, Mullol, Ferguson and Gevaert30,Reference Bachert, Han, Desrosiers, Hellings, Amin and Lee31 should be considered for intractable eosinophilic chronic rhinosinusitis.
This study had some limitations. First, not all patients with eosinophilic chronic rhinosinusitis could be followed up after FESS. There might have been some patients who did not visit our department, despite regular follow up being recommended. Second, sinonasal conditions and olfactory functions were not evaluated by examinations. Changes in the post-operative endoscopic appearance score were only evaluated four weeks after sinonasal topical steroid treatment, whereas weekly changes of symptoms using the nasal symptoms questionnaire were investigated. The correlations of symptoms (nasal symptoms questionnaire) and the sinonasal condition (post-operative endoscopic appearance score) needs to be further clarified, which could be done if all patients were seen every week. It is necessary for our department to improve the environment so that time-consuming olfactory tests can be performed easily. Finally, because this was a retrospective study conducted at one tertiary hospital, additional multicentre prospective studies are needed in the future.
Conclusion
In eosinophilic chronic rhinosinusitis, younger adult patients with severe rhinosinusitis tended to show recurrence after FESS. Sinonasal topical steroid treatment, which is effective for the treatment of nasal symptoms, appears to be one of the beneficial post-operative therapies for patients with eosinophilic chronic rhinosinusitis. Further, recent biological therapies that are indicated for intractable chronic rhinosinusitis with nasal polyps are expected to improve the quality of life of eosinophilic chronic rhinosinusitis patients.
Acknowledgements
The authors gratefully acknowledge the help of their technical assistants, Ms Yumi Kida and Ms Midori Tanide. This work was supported by Grants-in-Aid for Scientific Research (JSPS KAKENHI grant number: 20K09700) from the Japan Society for the Promotion of Science, and a Health Labour Sciences Research grant (H30-Nantitou(nan)-Ippan-016).
Competing interests
None declared