Introduction
Blast deafness is acute acoustic trauma induced by discontinuous impulse noise or an intense explosion. The inner ear is injured due to the large change in the apical peak of the frequency spectrum induced by the intense noise.Reference Gao, Chi and He1 The auditory brainstem response (ABR) threshold shift shows a process of slow-moving recovery after such trauma, which may be related to noise-induced cochlear oedema. In the early phase following such injury, scanning electron microscopy has identified oedema of the cuticular plate, collapse of the stereocilia of the inner and outer hair cells, and significant oedema of the hair cells; in the late phase, ciliary loss and hair cell death are seen.
Blast deafness can occur due to warfare, military training, mine explosions and powerful thunder. Research into the mechanisms, prevention and treatment of blast deafness is important in order to facilitate improved hearing protection.
Two general mechanisms are responsible for blast deafness: mechanicalReference Canlon2 and metabolic.Reference Puel, Ruel, Gervais d'Aldin and Pujol3 Mechanical damage occurs when movement of the basilar membrane is excessive, inducing detachment of the tectorial membrane, disconnection of the interciliary bridges and even rupture of the basilar membrane. Metabolic disorders have multiple origins: (1) ionic, involving an intracellular influx of potassium or calcium; (2) ischaemic; (3) excitotoxic, following an excessive release of glutamate; and (4) via the production of cochlear free radicals, which cause cell damage.
An important factor in blast deafness is the increased concentration of reactive oxygen species (such as aldehyde groups (e.g. malondialdehyde), ketones and hydroxide radicals), which result in damage to hair cells and spiral ganglion cells.Reference Cassandro, Sequino, Mondola, Attanasio, Barbara and Filipo4–Reference Seidman, Babu, Tang, Naem and Quirk6 Although it is unclear how noise induces the accumulation of reactive oxygen species, this may be related to a reduction in cochlear anti-oxidase activity. The concentration of malondialdehyde may reflect the degree of lipid peroxidation and thus indirectly indicate the extent of cell injury. The intra-cochlear malondialdehyde level increases after blast injury and decreases with prednisolone treatment in guinea pigs in vivo.Reference Liu7–Reference Karlidağ, Yalcin, Oztürk, Ustündağ, Gök and Kaygusuz9 Superoxide dismutase is the body's first line of defence against free radicals, and its concentration reflects the body's ability to clear reactive oxygen species.Reference McCord, Fridovich, Michelson, McCord and Fridovich10
In humans, glucocorticoids are used extensively in the management of inner ear pathology, such as sudden sensorineural hearing loss,Reference Plaza and Herráiz11 autoimmune inner ear diseaseReference Parnes, Sun and Freeman12 and Ménière's disease.Reference Garduňo-Anaya, Couthino de Toledo, Hinojosa-González, Pane-Pianese and Rios-Castaňeda13 Endogenous glucocorticoids have protective effects against noise-induced deafness.Reference Wang and Liberman14 Prednisolone has been shown to have a protective effect against blast injury in guinea pigs.Reference Sendowski, Abaamrane, Raffin, Cros and Clarencon15 When used to treat inner ear disorders, glucocorticoids are typically administered systemically.Reference Slattery, Fisher, Iqbal and Liu16, Reference Trune, Wobig, Kempton and Hefeneider17 Intratympanic administrationReference Takemura, Komeda, Yagi, Himeno, Izumikawa and Doi18 allows a higher local concentration of medication.
Animal studies have involved continuous injection of drugs via a miniature osmotic pump.Reference Sendowski, Abaamrane, Raffin, Cros and Clarencon15, Reference Shiwany, Seidman and Tang19 In the current study, we administered dexamethasone via the post-aurem route through the round window niche. Although dexamethasone is commonly used clinically at a concentration of 4 mg/ml, our study used 10 times that concentration. The pharmacokinetics of dexamethasone were assessed and its therapeutic effect on blast deafness evaluated, in order to investigate its mechanism of action against lipid peroxidation in this clinical setting.
Materials and methods
Animals and materials
This study used 74 adult, male, albino guinea pigs (supplied by the Experimental Animal Center, Shanghai, China) weighing approximately 250 g, with a normal Preyer's reflex.
All experimental procedures, including the care and use of animals, were approved by the laboratory animals department, Shanghai Medical College, Fudan University.
Dexamethasone 21-phosphate disodium salt (Sigma–Aldrich, Santa Clara, California, USA) was prepared at a concentration of 40 mg/ml in sterile water.
Experimental protocol
The experimental protocol for this study is shown in Figure 1. The experiment was divided into four parts.

Fig. 1 Outline of the experimental design. HPLC = high-performance liquid chromatography
In part one (i.e. preliminary experiment one), 10 guinea pigs were randomly divided into two groups: a saline group (n = 5) and a dexamethasone group (40 mg/ml, n = 5).
In part two (i.e. preliminary experiment two), another 10 animals were randomly divided into two groups: a noise plus saline group (n = 5) and a noise plus dexamethasone group (n = 5).
In part three (i.e. experiment one), 30 guinea pigs were randomly divided into two groups: a noise plus saline group (n = 15) and a noise plus dexamethasone group (n = 15).
In part four (i.e. experiment two), 24 guinea pigs were divided into eight groups of three each. Dexamethasone was administered via the round window niche; perilymph was then sampled 15, 30, 60, 120, 180, 240, 300 and 360 minutes afterwards.
Noise exposure
Within an anechoic room, the animals were exposed to acoustic trauma comprising 80 intense impulse noise events created using an electric spark generator (D-86: Jiangwan 1st Factory, Shanghai, China; Figure 2). Guinea pigs were placed in a custom-made frame 10 cm from the noise source. Impulse noise events had a peak value of 167 dB SPL, with a 2 second pulse interval and 0.5 millisecond pulse width.

Fig. 2 Apparatus used for impulse noise exposure.
Surgical procedure
The surgical procedure was performed one day after noise exposure, and is illustrated in Figure 3.

Fig. 3 The surgical procedure. (a, b) The guinea pig was anaesthetised generally and locally. (c, d) The bulla was exposed and opened under sterile conditions. (e) A gelatin sponge soaked with dexamethasone or saline was gently placed against the round window niche of each left cochlea. (f) Dental cement was used to close the bulla.
Before surgery, animals were anaesthetised with an intramuscular injection of Xylazine (25 mg; Pharma Chemical Plant, Nanjing, China) and ketamine (2 ml, 0.0008 ml/g). In addition, they were injected subcutaneously around the incision site with 0.5 ml of 0.5 per cent lidocaine hydrochloride for local anaesthesia.
The bulla was exposed and opened under sterile conditions. A gelatin sponge soaked with dexamethasone (40 mg/ml) or saline was gently placed on the round window niche of each left cochlea. Dental cement was used to close the bulla. The animals were left in quiet conditions for at least 30 minutes.
Auditory brainstem response measurement
In preliminary experiment one, ABR measurements were obtained before and one week after drug administration, to assess whether dexamethasone affected normal hearing. In preliminary experiment two, ABR was measured 6 hours after noise exposure and one, two, three and four weeks after drug administration, to determine the best point at which to assess the therapeutic effect of dexamethasone on blast deafness.
In experiment one, ABR measurements were obtained: before noise exposure (day 0), after noise exposure and before drug administration (day 1), and three weeks following drug administration (day 21). The first ABR measurement was performed to confirm normal hearing and to define baseline hearing for each animal. The threshold shift following drug administration was determined by comparing the ABR thresholds measured on day 21 versus day 1.
For ABR measurement, animals were anaesthetised using Xylazine 25 mg and ketamine 2 ml, 0.0008 ml/g. Stainless steel needle electrodes were placed subcutaneously at the vertex (the recording electrode), the left post-aurem (the ground electrode) and the right post-aurem (the reference electrode; see Figure 4). Animals were placed in an anechoic room and tones (0.1 millisecond click tone bursts, 13.3 times per second for repeated speed rate) were delivered to the left external auditory canal via tubes with adapters. Auditory brainstem responses were recorded with high and low pass filter settings of 150–1500 Hz. An average response was based on 500 sweeps, obtained at 5 dB intervals. Thresholds were determined by visual inspection of wave ш, at appropriate latency, verified three times (see Figure 5).

Fig. 4 Electrode placement for auditory brainstem response measurement.

Fig. 5 Determination of auditory brainstem response thresholds by visual inspection of wave ш.
Histological examination
Histological examinations were performed in experiment one. Six animals were sacrificed after the third ABR measurement. After injection of pentobarbital sodium (2 mg/ml), the cochleae were quickly removed. A solution of succinate dehydrogenase (containing 0.272 g of 0.2 M potassium dihydrogen phosphate, 6.447 g of 0.2 M dibasic sodium phosphate, 5.403 g of 0.2 M sodium succinate and 0.2 g of 0.1 per cent nitroblue tetrazolium) was infused through each cochlear window. The cochleae were placed in this solution at 37°C for 50 to 55 minutes and then fixed in 10 per cent formaldehyde for more than 8 hours at 4°C. They were then dissected under a binocular optical microscope to remove the organ of Corti from the apex to the base. The samples, mounted on a histological slide in glycerin, were observed under an optical microscope (magnification ×400). The prevalence of missing outer hair cells and inner hair cells was calculated.
Four animals from both the noise plus saline group and the noise plus dexamethasone group in experiment one were sacrificed, after ABR measurement. After deep anaesthesia by injection of pentobarbital sodium (2 mg/ml), the cochleae were quickly removed. Ten per cent formaldehyde was infused through the cochlear windows. The cochleae were fixed in the formaldehyde for over 8 hours and then decalcified in 10 per cent ethylene diamine tetra-acetic acid for seven days with one change per day. The cochleae were subsequently trimmed, oriented in agar and embedded in paraffin. Cochleae were sectioned at 10 µm thickness, and mid-modiolar sections were mounted on slides and stained with haematoxylin and eosin. The final specimens were observed under an optical microscope (see Figure 6).

Fig. 6 Photomicrograph of spiral ganglion cells from experimental group one animal. (H&E; ×400)
Malondialdehyde and superoxide dismutase concentration
Fourteen animals from both the noise plus saline group and the noise plus dexamethasone group in experiment one were sacrificed after deep anaesthesia by injection of pentobarbital sodium (2 mg/ml), as were seven normal, control animals. The cochleae were quickly removed and placed into pre-cooled phosphate-buffered saline. After removing the cochlear wall, the basement membrane and central axis of the left cochlea in each group were weighed, placed in a 1.5 ml centrifuge tube and ground into a cochlear homogenate in a quantity of saline nine times the weight of the tissue. The homogenate was then centrifuged at 4°C and 2000 revolutions/minute for 10 minutes; the supernatant represented the 10 per cent cochlear homogenate.
The malondialdehyde and superoxide dismutase concentrations in the homogenate were determined as instructed by the Kid directions (Jiancheng, Nanjing, China).
High-performance liquid chromatography
Dexamethasone was dissolved in 100 ml artificial perilymphReference Sendowski, Abaamrane, Raffin, Cros and Clarencon15 to yield a 120 µg/ml stock solution. A comparator curve was calculated by diluting the dexamethasone stock solution in mobile phase to final concentrations of 60, 30, 15, 7.5 and 6 µg/ml dexamethasone.
Twenty-four animals were anaesthetised with pentobarbital sodium (2 mg/ml) and 40 mg/ml of dexamethasone was administered via middle-ear injection. After 15, 30, 60, 120, 180, 240, 300 and 360 minutes, the cochleae were washed three times with a total amount of approximately 1 ml water. The animals were then sacrificed, and perilymph samples were collected by surgically removing the cochlea, opening the ear vesicle and extracting the cochlear perilymph fluid using a micro-injector via the round window membrane. Samples were immediately frozen at −20°C. Each cochlear perilymph fluid sample was taken from three cochleae.
High-performance liquid chromatography separation was conducted on a C18 column (4.6 × 150 mm, internal diameter 5 µm) at 20°C. The mobile phase was acetonitrile–ammonium sulphate (27:73 volume/volume) at a flow rate of 1.0 ml/minute, and the detection wavelength was 245 nm. The injection volume was 20 µl.
Statistical analysis
Auditory brainstem response threshold shifts for the experimental and control groups were compared using the chi-square test. The chi-square test was also used to compare the prevalence of missing outer hair cells in each animal, and the cochlear malondialdehyde and superoxide dismutase concentration in the experimental versus control groups. Differences were considered statistically significant when the p value was less than 0.05.
Results and analysis
Auditory brainstem response
In preliminary experiment one, there was no significant difference in the ABR click threshold shift between the two groups, either before (t = 0, p = 1.00) or after (t = 0.63, p = 0.54) drug administration. This suggests that dexamethasone at a concentration of 40 mg/ml had no effect on normal guinea pig hearing (see Figure 7).

Fig. 7 Auditory brainstem response (ABR) click threshold shift observed in the dexamethasone and saline groups (preliminary experiment group one), before (pre) and after (post) treatment. No statistically significant difference was seen between the two groups at either time point.
In preliminary experiment two, there was no significant difference in the ABR click threshold shift between the two groups one day after noise exposure. The threshold shift of the ABR click threshold shift one week, two weeks, three weeks and four weeks after saline administration was not significant in the noise plus saline group. In the noise plus dexamethasone group, the ABR click threshold shift decreased gradually, comparing the one-, two- and three-week results; however, there was no obvious difference between results at three and four weeks. Therefore, three weeks post-administration was chosen as the observation point for the study (see Figure 8).

Fig. 8 Auditory brainstem response (ABR) threshold shifts for the noise plus dexamethasone (dex) and noise plus saline groups in pre-experiment group two, and the ABR threshold shift difference between these two groups, following treatment administration. The ABR threshold shift difference increased gradually over weeks one to three, but was no larger on week four. Therefore, week three was chosen as the experimental observation point.
Figure 9 shows the ABR click threshold shift results for experimental group one before noise exposure, 1 day after noise exposure and three weeks after drug administration.

Fig. 9 Auditory brainstem response (ABR) click threshold shifts for the noise plus dexamethasone (dex) and noise plus saline groups in experimental group one, before and after noise exposure. Note that treatment was administered 1 day after noise exposure. A significant difference between the two groups' ABR threshold shifts was observed at both 1 and 21 days after noise exposure (p < 0.05).
Cochlear hair cell histology
In the experiment one animals, light microscopy revealed severe damage of the hair cells in the superior part of the basal turn and the inferior part of the second turn of the basal membrane; however, there was no damage or loss in the third and fourth turns. Basal membrane hair cell loss was more severe in the saline subgroup than in the dexamethasone subgroup. In the dexamethasone group, it was mainly the outer hair cells which were lost; few inner hair cells were lost (Figure 10a). In the saline group, few outer hair cells remained (Figure 10b). Hair cell loss in the dexamethasone versus saline subgroups is shown in Figure 10.

Fig. 10 Photomicrographs showing hair cell loss in the inferior part of the second cochlear turn. (a) In the dexamethasone group, it was mainly the outer hair cells which were lost; few inner hair cells were lost. (b) In the saline group, few outer hair cells remained. (Succinate dehydrogenase staining; ×400)
In experiment one animals, hair cell loss in the basal turn, the second turn and overall was significantly greater in the saline group than in the dexamethasone group (p < 0.05). However, there was no significant difference in hair cell loss in the third and apical turns, comparing these two groups (p > 0.05; see Table I).
Table I Percentage hair cell loss in each cochlear turn

Data refer to experimental group one, and represent means ± standard deviations (%) unless otherwise specified. Dex = dexamethasone
Spiral ganglion morphology
On light microscopy 21 days after drug administration in experiment one animals, there was no significant difference in spiral ganglion cell counts in the inferior part of the second turn and the superior part of the basal turn, comparing the dexamethasone and saline subgroups (Figure 11).

Fig. 11 Photomicrographs showing spiral ganglion cells in the (a) inferior second turn and (b) superior basal turn in the dexamethasone group; and in the (c) inferior second turn and (d) superior basal turn in the saline group. (H&E; ×400)
Malondialdehyde and superoxide dismutase concentration
Table II shows the malondialdehyde and superoxide dismutase concentrations in experiment one animals' cochleae 21 days after treatment, and also in seven untreated animals (used as controls).
Table II Malondialdehyde and superoxide dismutase concentration 21 days after treatment
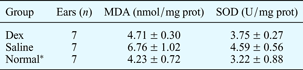
Data refer to experimental group one, and represent means ± standard deviations unless otherwise specified. *Seven normal, untreated animals used as controls. MDA = malondialdehyde; prot = protein; SOD = superoxide dismutase; dex = dexamethasone
Malondialdehyde and superoxide dismutase concentration were significantly greater in the dexamethasone group compared with the normal group (p < 0.05). The difference in malondialdehyde and superoxide dismutase concentration between the saline and normal groups was not significant (p > 0.05).
High-performance liquid chromatography
Following round window membrane dexamethasone administration in experiment two animals, high-performance liquid chromatography measurements indicated that the peak concentration of dexamethasone in cochlear perilymph was 5330.522 µg/ml. The cochlear perilymph dexamethasone concentration decreased gradually over time; 360 minutes after drug administration, it was 299.7966 µg/ml. The plotted curve for cochlear perilymph dexamethasone concentration versus time had an equation of Y = 0.00005C + 0.447, with R = 0.9998 and a linearity range of 0.1–10 mg/ml (Table III). This curve is shown in Figure 12.

Fig. 12 Cochlear perilymph dexamethasone concentration versus time, in experiment two animals.
Table III Perilymph dexamethasone level over time*

* After topical application of dexamethasone (dex) to round window niche.
Discussion
Determination of drug administration route and concentration
Mini-osmotic pumps connected to a cannula are often used to infuse drugs into the scala tympani in animal research models, in order to observe the therapeutic effect. This administration route has the benefit of achieving high drug concentrations in the inner ear, without systemic effects.
A study on guinea pigs has investigated the effects of transtympanic dexamethasone injection on cochlear blood flow (assessed with laser Doppler flowmetry), auditory sensitivity (assessed by ABR) and histology.Reference Shiwany, Seidman and Tang19 A significant increase in cochlear blood flow was observed within 30 seconds of injection, to a mean of 129.26 per cent, without any significant change in auditory sensitivity. This increase in cochlear blood flow was sustained, and did not return to baseline until at least 1 hour after drug application. No histological changes were observed. The author concluded that transtympanic steroid application was not likely to be detrimental to the inner ear, and also that the increased blood flow may indicate a possible mechanism accounting for the pharmacological effects of steroids in the inner ear.
Our study findings suggest that dexamethasone has no effect on auditory function in normal guinea pigs. We chose to administer dexamethasone via the round window membrane, because of the ease and potential clinical application of this route. Other authors have used direct delivery routes other than the round window, sometimes with slow- or sustained-release formulations.Reference Banerjee and Parnes20 In these studies, the experimental drug was injected into the middle ear directly, to the required volume. In a preliminary experiment conducted prior to the current study, we compared the round window and middle-ear administration routes, and found that the latter could result in more inflammation in the middle ear and even the inner ear.
When considering drug administration via the round window membrane, one major question is whether the drug is able to permeate the round window membrane and enter the perilymph to a sufficient degree to reach a therapeutic concentration. The round window membrane is considered the principal route of entry for transtympanically injected drugs. It is a semi-filtration membrane which has different filtration properties for different substances. The permeability of the round window membrane to different substances is affected by several factors, including the substance's molecular size, configuration, concentration, liposolubility and electric charge, and also the round window thickness. Previous studies have noted that both transforming growth factor-α and the GJB2 R75W gene are able to pass through the mammalian round window membrane.Reference Witte and Kasperbauer21, Reference Maeda, Fukushima, Kawasaki, Nishizaki and Smith22 The relative molecular mass of dexamethasone is 516.41 Da, smaller than that of transforming growth factor-α and the GJB2 R75W gene.
In Li and colleagues' study,Reference Li, Gong, Chen, Lu and Wang23 a Waters 600 high-pressure liquid chromatograph was used to investigate the pharmacokinetics of dexamethasone infiltration of the round window membrane. These authors verified that dexamethasone at a concentration of 100 g/l could pass through the round window membrane quickly, achieving a peak value of 310.6 g/l 60 minutes after administration. The concentration then decreased gradually to 35 mg/l 300 minutes later.
In our study, a gelatin sponge soaked with dexamethasone at a concentration of 40 mg/ml was placed on the round window niche. Because of the location of the gelatin sponge, dexamethasone had sufficient time to permeate into the cochlear perilymph, and achieved a high concentration as predicted. High-performance liquid chromatography indicated that the highest perilymph dexamethasone concentration was 5330.522 µg/ml, and was still 299.797 µg/ml 6 hours after administration.
Other authors have found that histamine facilitates permeation of dexamethasone into the cochlear perilymph.Reference Chandrasekhar, Rubinstein, Kwartler, Gatz, Connelly and Huang24
In the clinic, sudden sensorineural hearing loss is often treated with intratympanic administration of dexamethasone at a concentration of 24 mg/ml.Reference Haynes, O'Malley, Cohen, Watford and Labadie25
The protective effect of dexamethasone against noise-induced trauma has been assessed in guinea pigs.Reference Takemura, Komeda, Yagi, Himeno, Izumikawa and Doi18 Animals were administered dexamethasone (at 1, 10, 100 and 1000 ng/ml) and artificial perilymph directly into the scala tympani, via a mini-osmotic pump, and then exposed to 120 dB SPL octave band noise, centred at 4 kHz, for 24 hours. Seven days after noise exposure, results in dexamethasone-treated animals indicated a dose-dependent reduction in noise-induced outer hair cell loss (significant at 1, 10 and 100 ng/ml dexamethasone) and a similar attenuation of noise-induced ABR threshold shifts (significant at 100 ng/ml dexamethasone), compared with control animals receiving artificial perilymph.
In our study, we were able to achieve a higher peak dexamethasone concentration in the cochlear perilymph (i.e. 5330.522 µg/ml) than that reportedly achieved in previous studies (100 ng/ml).Reference Takemura, Komeda, Yagi, Himeno, Izumikawa and Doi18
The cochlear perilymph dexamethasone concentration is also influenced by the timing of drug application to the round window membrane. A recent studyReference Chang, Eastwood, Sly, James, Richardson and O'Leary26 examined the dosage and timing of dexamethasone applied to the round window in order to optimise hearing protection. A reduced application time was needed for higher steroid concentrations: 20 per cent dexamethasone applied for 30 minutes achieved similar levels of hearing protection to 2 per cent dexamethasone applied for 60 minutes. Prior to cochlear implantation, a longer duration of dexamethasone application to the round window resulted in improved hearing protection; this waiting time could be reduced by increasing the steroid concentration. These results indicate that the diffusion of dexamethasone into the cochlea is the prime determinant of the degree of hearing protection achieved. In our study, a gelatin sponge was used to apply dexamethasone for a sufficient time period to allow permeation through the round window membrane into the cochlear perilymph.
Therapeutic effect of dexamethasone on blast deafness
Exposure to high intensity noise can cause irreversible hearing damage, as a result of direct mechanical or secondary metabolic effects. In China and various other countries, the recommended treatment for noise-induced hearing loss consists of nursing in a silent environment, intravenous injection of glucocorticoid with or without vasodilator substances, and hyperbaric oxygen therapy. A protective effect of glucocorticoid has been demonstrated by Henry et al.Reference Henry27 In the current study, guinea pigs were administered either dexamethasone or saline following noise exposure. Noise-induced hearing loss was reduced in the dexamethasone group compared with controls. This protective effect has also been observed for methylprednisolone (another glucocorticoid), by Zhou et al.,Reference Zhou, Zheng, Shen, Zhang and Yang28 following intratympanic administration of methylprednisolone or saline in guinea pigs. Furthermore, a protective effect has been observed for endogenous glucocorticoids: activation of the hypothalamus–pituitary–adrenal axis is associated with reduced threshold shifts after acoustic trauma.Reference Wang and Liberman14
Current treatment for acute acoustic trauma is based on systematic or local glucocorticoid administration. A study by Abaamrane et al. Reference Abaamrane, Raffin, Gal, Avan and Sendowski29 compared three noise-induced hearing loss treatments applicable in humans: magnesium for seven days or one month, or conventional methylprednisolone therapy. Three months after impulse noise trauma, one-month magnesium treatment preserved more hair cells than the two other treatments. Although methylprednisolone accelerated functional recovery, compared with magnesium, its long-term advantage remained moderate. In this study, several exploratory methods were used to evaluate the efficacy of dexamethasone infiltration through the round window membrane, following impulse noise trauma.
In the current study, a particularly damaging noise trauma was applied: 80 impulses with 167 dB SPL peak value, sufficient to induce permanent hearing loss and intensive, irreversible hair cell loss in guinea pigs (as previously described by Zhou et al.).Reference Zhou, Zheng, Shen, Zhang and Yang28 Baseline auditory thresholds were determined in all animals before noise exposure and before treatment. In experimental group one, the study and control animals had comparable auditory function before noise exposure, and before treatment. After noise exposure, the ABR click threshold shift decreased by 50 to 80 dB in both the study and control subgroups, and light microscopy indicated hair cell loss especially in the superior part of the basal turn and the inferior part of the second turn. Outer hair cell loss was more severe than inner hair cell loss. These changes in hearing function and hair cell morphology concur with the formation of a permanent threshold shift. These effects have already been observed in guinea pigs with noise-induced hearing loss treated with intracochlear methylprednisolone administration.Reference Sendowski, Abaamrane, Raffin, Cros and Clarencon15
Mechanism of glucocorticoid effect following blast deafness
Glucocorticoids are used extensively in the treatment of disease, because of their intensive anti-inflammatory action and other therapeutic effects. Glucocorticoids such as dexamethasone not only protect normal hair cells but also inhibit further injury of damaged hair cells. In our experimental group one, hair cell injury observed three weeks after treatment was significantly less in the dexamethasone group compared with the control group, indicating that dexamethasone has a therapeutic effect following intense acoustic trauma. In addition, we observed no significant difference in the corresponding spiral ganglion cells, comparing the dexamethasone and control groups, suggesting that noise may not damage the spiral ganglion cells.
The exact manner by which dexamethasone improves noise-induced hearing loss is still unknown, but several mechanisms may be possible. At the cellular level, glucocorticoids exert their effects by binding to the glucocorticoid receptor, a transcription factor which belongs to the nuclear receptor superfamily and is capable of regulating several genes. Glucocorticoid receptors have been demonstrated within the inner ear in rodents and humans, in the spiral ligament, spiral ganglion cells and spiral limbus, and to a lesser extent in the stria vascularis and the inner and outer hair cells of the organ of Corti, amongst other sites.Reference Xu30 The administration of RU486, a glucocorticoid receptor antagonist, to noise-traumatised mice antagonised the protective effect of methylprednisolone. Thus, the protective effect of this drug in the inner ear appears to be mediated by the binding of glutathione reductase.Reference Hirose, Tabuchi, Oikawa, Murashita, Sakai and Hara31 Tahera et al. Reference Tahera, Meltser, Johansson, Bian, Stierna and Hansson32 demonstrated that glucocorticoid can directly modulate hearing sensitivity following moderate acoustic trauma resulting in a 10–30 dB hearing loss. Importantly, this study demonstrated that nuclear factor-κB (NF-κB) is involved in the glutathione reductase mediated signalling pathway in the inner ear. These authors concluded that down-regulation of endogenous glucocorticoid production and increased nuclear translocation within the cochlea resulted in reduced acoustic trauma. Mifepristone, another glucocorticoid receptor antagonist, may influence the inner hair cells and the afferent nerve fibres beneath the inner hair cells, without affecting the outer hair cells.Reference Mori, Fujimura, Yoshida and Suzuki33 Thus, the evidence suggests that glucocorticoids can play an important therapeutic role in treating hearing impairment following acoustic trauma.
Karlidag et al. evaluated the efficacy of methylprednisolone administered (40 mg/kg intramuscular) to guinea pigs prior to noise exposure. Malondialdehyde levels within cochlear tissue were used as a marker of oxidative stress. However, in this study methylprednisolone was found to have no significant effect on the malondialdehyde concentration increase observed after noise exposure. This apparent discrepancy, compared with previous findings, may be explained by differing routes of methylprednisolone administration.
During lipid peroxidation, a significant quantity of aldehydes (e.g. malondialdehyde) are produced by β-schizolysis of the lipid group. The extracellular malondialdehyde concentration reflects the degree of injury caused by oxygen free radicals attacking cell membranes;Reference Wu, Chen, Sung, Jean, Shih and Shyu34 thus, malondialdehyde concentration can be used as an indicator of the degree of lipid peroxidation. Uhler et al. Reference Uhler, Frim, Pakzaban and Isacson35 found that high-dose methylprednisolone could prevent membrane lipid peroxidation injury induced by free radicals in vivo, and that it had an anti-oxidant effect. Dexamethasone may decrease ischaemia reperfusion injury by lowering the malondialdehyde concentration.Reference Koc, Akdemir, Karakücük, Oktem and Menkü8 Yamane et al. found that superoxide anions were formed in the luminal membranes of guinea pigs following exposure to noise at 120–125 dB for 3 hours, and were also expressed in the stria vascularis after noise exposure.Reference Yamane, Nakai, Takayama, Konishi, Iguchi and Nakagawa36 The greater the noise intensity, the greater the expression of hydroxide radicalsReference Ohinata, Miller, Altschuler and Schacht37, Reference Ohlemiller, Wright and Dugan38 and malondialdehydeReference Kaygusuz, Ozturk, Ustundağ and Yalcin39 in guinea pig cochleae. Dexamethasone prevents lipid peroxidation through activating anti-oxidases such as glutathione reductase and superoxide dismutase.Reference Mori, Fujimura, Yoshida and Suzuki33
In experiment two, the malondialdehyde concentration in the dexamethasone group was greater than that in the normal group but less than that in the saline group. This suggests that oxygen free radical activity in the dexamethasone group was lower than that in the saline group. We conclude that dexamethasone decreased the amount of oxygen free radicals induced by acoustic trauma, and also accelerated the metabolism and clearing of oxygen free radicals by reinforcing anti-oxidation capacity, thus reducing cellular injury and protecting the guinea pigs from hearing damage.
Superoxide dismutase is an effective oxygen free radical scavenger and can alleviate noise-induced hearing loss. It clears reactive oxygen species and thus helps balance oxidative conditions. Certain pathological conditions strengthen the lipid peroxidation process; this may trigger a compensatory increase in superoxide dismutase synthesis. We found greater superoxide dismutase activity in both the saline and dexamethasone groups, compared with the normal group; however, this value was greatest in the saline group. This suggests that dexamethasone may strengthen the anti-oxidative capability of the cochlea, and thus enable improved hearing following acoustic trauma. Further study is required to confirm this last point.
Methylprednisolone (delivered via intrathecal injection) has been found to be beneficial for the treatment of ischaemic spinal cord injuries. Intratympanic dexamethasone delivery increases the cochlear blood flow. These results suggest that glucocorticoids may protect hair cells from ischaemic and hypoxic injury through increasing the cochlear blood flow.Reference Shiwany, Seidman and Tang19, Reference Wu, Chen, Sung, Jean, Shih and Shyu34
Nagashima et al. Reference Nagashima, Sugiyama, Yoneyama and Ogita40 observed that systemic administration of dexamethasone led to a significant increase in glutathione concentration in the cochlear modiolus, 2 to 24 hours after injection.
Under normal conditions, the inner ear possesses remarkably stable homeostatic mechanisms enabling maintenance of the functional integrity of the inner ear fluid. Any disturbance that disrupts homeostasis may have short- and long-term effects on the cellular function of the auditory system. Traumatic noise exposure has been demonstrated histopathologically to destroy auditory hair cells (especially outer hair cells), to contribute to collapse of the organ of Corti, and to impair cochlear circulation.Reference Juhn, Hunter and Odland41
• Glucocorticoids are used extensively in the clinical management of inner ear pathology, including sudden sensorineural hearing loss
• The exact mechanism by which dexamethasone improves noise-induced hearing loss is still unknown
• Dexamethasone has therapeutic effects on blast deafness in guinea pigs by protecting hair cells and accelerating hearing recovery; one probable mechanism is action against oxygen free radical toxicity
Recently, the general caspase inhibitor N-t-Boc-Asp(OMe)-fluoromethylketone has been found to prevent tumour necrosis factor α induced auditory hair cell death. Tumour necrosis factor α is a pro-inflammatory cytokine. A significant reduction in the efficacy of dexamethasone otoprotection against tumour necrosis factor α ototoxicity has been observed when the following substances, structures and cellular events are blocked: (1) glucocorticoid receptors; (2) phosphatidylinositol 3-kinase; (3) activation of protein kinase B; and (4) NF-κB-1. Dexamethasone protects hair cells against tumour necrosis factor α apoptosis in vitro via activation of phosphatidylinositol 3-kinase, protein kinase B and NF-κ B-1 signalling.Reference Haake, Dinh, Chen, Eshraghi and Van De Water42 The ototoxicity of tumour necrosis factor α may be mediated via up-regulation of Bax and tumour necrosis factor receptor 1 expression, as well as via an increase in the Bax/Bcl-2 ratio. Dexamethasone protects the organ of Corti against tumour necrosis factor α ototoxicity by up-regulating Bcl-2 and Bcl-xl expression, and by inhibiting tumour necrosis factor α induced increases in Bax, tumour necrosis factor receptor 1, and the Bax/Bcl-2 ratio. These results support the use of local dexamethasone treatment to conserve hearing following acoustic trauma.
Conclusion
From these results, we conclude firstly that dexamethasone applied topically to the round window niche has a therapeutic effect on guinea pigs with signs of blast deafness, by protecting hair cells and accelerating hearing recovery.
Secondly, impulse noise increases the concentration of malondialdehyde and superoxide dismutase within cochlear tissues, and the application of dexamethasone after noise exposure decreases this rise. This suggests that one of the mechanisms by which dexamethasone protects guinea pig hearing following blast deafness probably involves action against oxygen free radical toxicity.
Thirdly, we conclude that dexamethasone can permeate the round window membrane and reach the cochlear perilymph, where it can attain a high concentration for a relatively long period.
Acknowledgements
The authors thank Professors Li Zhong-dong and Tea Wang Yi for their assistance with high-performance liquid chromatography, and Professors Liang Zhen-fu and Tang Zhi-wen for providing the noise generator. All authors read and approved the final manuscript. The authors would like to acknowledge receipt of the following grants (1) NSFC grant 81028003/H1305; (2) Shanghai Scientific & technological Basic Research Important Item 10JC1402500; (3) Shanghai Scientific & technological International cooperating project 10410700800.

















