Introduction
Head and neck malignancies frequently produce pulmonary metastases, which are associated with poor prognosis as they are considered to be systemic disease.Reference Nibu, Nakagawa, Kamata, Kawabata, Nakamizo and Nigauri1–Reference Yamagata, Onizawa, Otsuka and Yoshida3 The incidence of clinically detected distant metastases from head and neck cancers ranges from 5.5 to 40 per cent,Reference Wedman, Balm, Hart, Loftus, Hilgers and Gregor2, Reference Finley, Verazin, Driscoll, Blumenson, Takita and Bakamjian4, Reference Daiko, Nagai, Yoshida, Nishimura, Hishida and Ebihara5 and the rate of pulmonary metastases from these cancers is reported to range between 6.0 and 9.1 per cent.Reference Yamagata, Onizawa, Otsuka and Yoshida3, Reference Finley, Verazin, Driscoll, Blumenson, Takita and Bakamjian4 The lung is the major target organ for distant metastasis from head and neck malignancies;Reference Nibu, Nakagawa, Kamata, Kawabata, Nakamizo and Nigauri1, Reference Wedman, Balm, Hart, Loftus, Hilgers and Gregor2, Reference Shiono, Kawamura, Sato, Okumura, Nakajima and Yoshino6 however, limited information is available on the prognostic factors associated with pulmonary metastasectomy for head and neck based malignancies.
The current study assessed the correlation between survival outcome and the clinicopathological tumour characteristics of 21 head and neck cancer patients with newly diagnosed and consequently resected pulmonary metastases. The goal was to identify factors that could predict the survival rates of head and neck cancer patients with resected pulmonary metastases. We assessed the accuracy of the evaluation in determining the most appropriate treatment for pulmonary metastases from head and neck malignancies, and compared the results with the clinical and histopathological characteristics of these tumours.
Materials and methods
Between 2005 and 2014, 21 patients with pulmonary metastases from primary head and neck malignancies were surgically treated at our institution. A metastasectomy was performed in all cases; patients were referred for metastasectomy only if there were no signs of locoregional recurrence, the metastases were resectable and the pulmonary condition was amenable to surgery after evaluation by thoracic surgeons. All patients in this study had histological diagnoses of malignant head and neck tumours, and none had any other distant metastases.
Factors affecting the survival rate, such as staging, pathological type, treatment procedures and disease-free interval, were analysed. The disease-free interval was calculated from the initial date of definitive treatment of the head and neck cancer to the date of diagnosis of the pulmonary metastasis with radiological examinations. Survival time was defined as the interval from the date of initial resection of lung tumours to the date of the last follow up or death.
Overall survival was calculated using the Kaplan–Meier method. The log-rank test was used to compare survival between different groups of patients. A p value of 0.05 or less was considered statistically significant. The statistical software package Stata version 11 (Stata, College Station, Texas, USA) was used to analyse the data.
Results
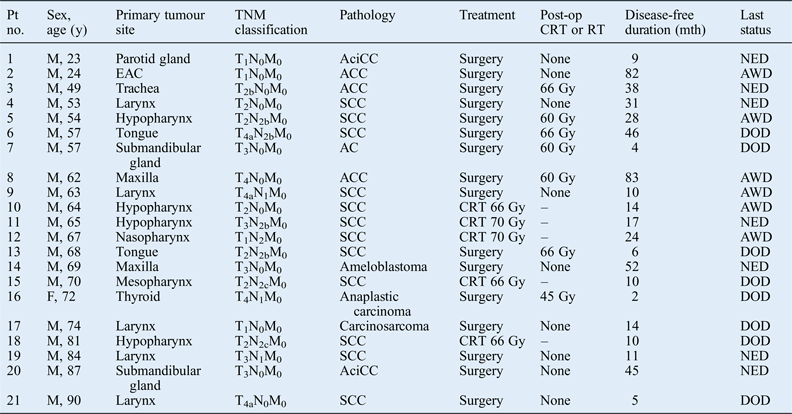
Twenty males and 1 female with pulmonary metastases arising from head and neck tumours were treated at our institution over a 10-year period, from 2005 to 2014. The median patient age was 63 years (range, 23–90 years). Primary tumour factors, with details of the site of occurrence and histology, are shown in Tables I–III.
Table I Tumour data*

*For 21 head and neck cancer patients with pulmonary metastases. Pt no. = patient number; y = years; TNM = tumour–node–metastasis; post-op = post-operative; CRT = chemoradiotherapy; RT = radiotherapy; mth = months; M = male; AciCC = acinic cell carcinoma; NED = no evidence of disease; EAC = external auditory canal; ACC = adenoid cystic carcinoma; AWD = alive with disease; SCC = squamous cell carcinoma; DOD = died of disease; AC = adenocarcinoma; F = female
Table II Summary of clinicopathological characteristics*

*For 21 head and neck cancer patients with pulmonary metastases. †Median age of 63 years (range, 23–90 years).
Table III Primary disease treatment methods*

*For 21 head and neck cancer patients with pulmonary metastases. Post-op = post-operative; CRT = chemoradiotherapy; RT = radiotherapy
The locations of the primary tumour were as follows: larynx (n = 5), hypopharynx (n = 4), salivary gland (n = 3), tongue (n = 2), maxillary sinus (n = 2), nasopharynx (n = 1), mesopharynx (n = 1), external auditory canal (n = 1), thyroid (n = 1) and trachea (n = 1).
Pre-therapeutic staging evaluation included radiological studies in all patients, using chest radiographs, computed tomography, and magnetic resonance imaging of the head and neck. At initial presentation, the primary tumours were classified as follows: T1 (n = 4), T2 (n = 7), T3 (n = 5) and T4 (n = 5) using the tumour–node–metastasis (TNM) classification.Reference Sobin, Gospodarowicz and Wittekind7 Two patients (9.5 per cent) had stage I disease, five (23.8 per cent) had stage II disease, six (28.6 per cent) had stage III disease and eight (38.1 per cent) had stage IV disease. Eleven patients (52.4 per cent) had no lymph node metastasis; however, three (14.3 per cent) had N1 and seven (33.3 per cent) had N2 lymph node metastasis.
The histological diagnosis in most patients (n = 12, 57 per cent) was squamous cell carcinoma (SCC). Three patients (14.3 per cent) had adenoid cystic carcinoma, two (9.5 per cent) had acinic cell carcinoma, and there was one case (4.8 per cent) each of adenocarcinoma, carcinosarcoma, anaplastic carcinoma and ameloblastoma.
The primary tumours were treated as shown in Table III. Sixteen patients (76.2 per cent) underwent surgical therapy; surgical resection with consecutive chemoradiotherapy or radiotherapy was performed in eight patients (38.1 per cent). Five patients (23.8 per cent) received chemoradiotherapy or radiotherapy only. The irradiation dose ranged from 66 to 70 Gy, administered in fractions of 2 Gy/day.
The diagnosis of pulmonary metastasis was made after a mean interval of 25.8 months (range, 2–83 months). Only one patient (4.8 per cent) exhibited signs of locoregional recurrence. In 20 patients, the lungs were the only site of distant metastases. Sixteen patients underwent one metastasectomy, four underwent two procedures and one underwent three.
The Kaplan–Meier survival analysis data are presented in Figures 1–6. The 5- and 10-year overall survival rates of the patients were 67.0 per cent and 55.0 per cent, respectively (Figure 1). The five-year overall survival rate after metastasectomy was 46.0 per cent (Figure 2). The 5-year overall survival rates of patients with a disease-free interval of longer or shorter than 24 months were 75.0 per cent and 19.0 per cent, respectively (p = 0.0234) (Figure 3). The post-metastasectomy 5-year overall survival rates of patients who were less than 60 years or 60 years or older were 80.0 per cent and 0.0 per cent, respectively (p = 0.0552). Although there was no significant difference between the age groups, older patients tended to have a worse prognosis than younger patients (Figure 4). Patients with SCC versus other histological groups had five-year overall survival rates of 49.0 per cent and 41.0 per cent, respectively (Figure 5); again, there was no significant difference (p = 0.62). There was also no significant difference in the 5- and 10-year overall survival rates between patients who underwent chemoradiotherapy or radiotherapy after surgery and those who did not (50.0 per cent and 35.0 per cent respectively; p = 0.3382), although the former group tended to have an advantage (Figure 6).

Fig. 1 Kaplan–Meier survival analysis: overall survival of the entire case series.

Fig. 2 Kaplan–Meier survival analysis: overall survival of the patients after pulmonary metastasectomy.

Fig. 3 Kaplan–Meier survival analysis: overall survival according to the disease-free interval (DFI).

Fig. 4 Kaplan–Meier survival analysis: overall survival according to age (less than 60 years vs 60 years and older).

Fig. 5 Kaplan–Meier survival analysis: overall survival of patients with squamous cell carcinoma (SCC) versus those with other disease subtypes.

Fig. 6 Kaplan–Meier survival analysis: overall survival in patients who underwent adjuvant chemoradiotherapy (CRT) versus those who did not.
At the time of writing, seven patients (33.3 per cent) were alive with no evidence of disease and six (28.6 per cent) were with disease. Eight patients (38.1 per cent) died; seven died of their original disease and one died of an unrelated disease. The information on the survival status of the patients was obtained after a mean follow-up period of 29.6 months (range, 1–91 months) after metastasectomy.
Discussion
Because the most frequent site of distant metastasis in patients with head and neck malignancies is the lung,Reference Ferlito, Shaha, Silver, Rinaldo and Mondin8–Reference Papac10 pulmonary metastases are considered to be a major problem after definitive treatment of these malignancies. It is often difficult to distinguish pulmonary metastatic lesions from primary lung cancers in patients with head and neck cancer because of the similarities in histopathological and radiographical appearance. Therefore, no universally accepted procedure or surgical approach has been adopted to treat these tumours.Reference Nibu, Nakagawa, Kamata, Kawabata, Nakamizo and Nigauri1–Reference Yamagata, Onizawa, Otsuka and Yoshida3
In this study, the five-year overall survival rate in patients diagnosed with pulmonary metastases from head and neck malignancies was 46.0 per cent. Prognostic factors were analysed, including disease-free interval, age, pathology and adjuvant chemoradiotherapy. Only a disease-free interval of less than 24 months was determined to be a significant factor, negatively affecting survival, according to univariate analysis. Two other investigations also found that a disease-free interval of less than 24 months was associated with poor prognosis,Reference Daiko, Nagai, Yoshida, Nishimura, Hishida and Ebihara5, Reference Liu, Labow, Dang, Martini, Bains and Burt11 whereas other studies found that a disease-free interval of less than 12 months was a poor prognostic factor.Reference Nibu, Nakagawa, Kamata, Kawabata, Nakamizo and Nigauri1, Reference Wedman, Balm, Hart, Loftus, Hilgers and Gregor2, Reference Finley, Verazin, Driscoll, Blumenson, Takita and Bakamjian4, Reference Chen, Sonobe, Sato, Fujinaga, Shoji and Sakai12, Reference Yamazaki, Shodo, Ueki, Matsuyama and Takahashi13 A short disease-free interval therefore appears to be predictive of a negative outcome.
• A retrospective review of 21 patients who underwent resection of pulmonary metastases of primary head and neck malignancies was performed
• Patients with short disease-free intervals, and possibly those who are older than 60 years, should be categorised as having severe disease
• Pulmonary metastases from head and neck malignancies are potentially curable by surgical resection
Our study also revealed that patients aged 60 years or older had markedly (although not significantly) poorer prognoses than younger patients.
The histological subtype of the primary tumours was not a significant prognostic factor. Squamous cell carcinomas are the most frequent histological subtype of head and neck tumours, and they are generally more aggressive and are associated with worse prognoses than other subtypes.Reference Wedman, Balm, Hart, Loftus, Hilgers and Gregor2, Reference Ferlito, Shaha, Silver, Rinaldo and Mondin8, Reference Geurts, Nederlof, van den Brekel, van't Veer, de Jong and Hart14, Reference Geurts, Balm, van Velthuysen, van Tinteren, Burgers and van Zandwijk15 Although SCC patients may generally fare worse than those with other cancer subtypes, there are numerous long-term survivors who have undergone resections.
Finally, we did not detect a significant survival advantage for adjuvant chemoradiotherapy after metastasectomy.
Our results indicate that pulmonary resection should be considered in head and neck cancer patients with pulmonary metastases, regardless of whether the tumours are SCCs or other subtypes, if the patients otherwise meet the criteria for pulmonary resection. Our data strongly suggest that pulmonary resection after primary head and neck cancer treatment is safe and increases patients' long-term survival. Additional studies are required to determine the optimal treatment method for these metastases.
Conclusion
This study reports the survival outcomes of patients who underwent surgical treatment for pulmonary metastases of head and neck malignancies at our hospital. The results suggest that complete pulmonary metastasectomy ought to be the treatment of choice. Patients with a disease-free interval of longer than 24 months have more favourable prognoses, and patients who are less than 60 years old may also have a survival advantage. However, further studies and meta-analyses are required to confirm these observations owing to the small number of patients in our series.
Acknowledgement
The authors thank Dr Kazuya Suzuki (former Associate Professor in the First Department of Surgery, Hamamatsu University School of Medicine, Japan) for his instruction with surgery and with this manuscript.