Introduction
Soft tissue head and neck sarcoma accounts for less than 1 per cent of all head and neck neoplasms.Reference Edge, Byrd, Compton, Fritz, Greene and Trotti1 These are both histologically and clinically heterogeneous tumours. Their phenotypes range from relatively indolent, locally destructive tumours (e.g. atypical lipomatous tumour), to locally aggressive neoplasms with metastatic potential (e.g. angiosarcoma).Reference Edge, Byrd, Compton, Fritz, Greene and Trotti1,Reference Zagars, Ballo, Pisters, Pollock, Patel and Benjamin2 Advances in immunohistochemical and molecular techniques have revolutionised diagnostic accuracy and thereby improved clinical outcomes through tumour-type-specific intervention. Surgery is the traditional mainstay of treatment. Achieving wide margins is crucial for optimal treatment.Reference Mattavelli, Miceli, Radaelli, Mattavelli, Cantù and Barisella3 This is, however, influenced by the infiltrative nature of the tumour, and the proximity of crucial structures found within the head and neck.Reference Mattavelli, Miceli, Radaelli, Mattavelli, Cantù and Barisella3
There are few published case series of head and neck soft tissue sarcoma worldwide. Therefore, evidence on which to base any treatment decision is limited. With this in mind, we sought to evaluate our experience of adult head and neck soft tissue sarcoma patients presenting to our multidisciplinary team (MDT) over the last 15 years, with particular attention to specific prognostic features.
The World Health Organization (WHO) classifies most soft tissue sarcomas according to the presumptive tissue of origin. In February 2013, the latest edition of the WHO Classification of Tumours of Soft Tissue and Bone was released.Reference Fletcher, Bridge, Hogendoorn and Mertens4,Reference Noujaim, Thway, Sheri, Keller and Jones5 In addition to benign and malignant categories, this publication defined two new intermediate sarcoma categories: ‘locally aggressive, never metastasising’ and ‘locally recurrent, rarely metastasising’. The prototypical lesion of the former category is desmoid (aggressive) type fibromatoses, whilst the latter category contains numerous rare entities, many described over the last two decades. These tumour types show unpredictable behaviour in-between that of malignancy and benignity, and are thus not graded or staged.
The WHO recommended grading malignant soft tissue sarcomas as per the French Fédération Nationale des Centres de Lutte Contre le Cancer (‘FNCLCC’) classification, also known as the Trojani system. This is a three-tier system, in which grade one is regarded as low grade, and grades two and three as high grade. This system has shown an increased ability to predict distant metastasis and disease-specific mortality.Reference Guillou, Coindre, Bonichon, Nguyen, Terrier and Collin6
Materials and methods
Ethical considerations
Permission to conduct the study was sought from the relevant National Health Service trusts to ensure information governance standards were adhered to. As this was a retrospective case review, formal ethical approval was not required.
Patient selection
Since 1997, one of the authors (TM) has maintained a prospective database of all patients diagnosed with soft tissue head and neck sarcoma diagnosed by the regional sarcoma MDT.
The present study comprised all adult (aged over 18 years old) cases of malignant soft tissue sarcoma and all WHO intermediate type tumours diagnosed between 1997 and 2012. Cutaneous spindle cell scalp tumours, often termed ‘cutaneous sarcoma’, were specifically excluded because of their uncertain histogenesis.Reference O'Neill, Bilsky and Kraus7 Primary tumours of bone were also excluded.
Malignant tumours where grade is predictive of biological behaviour (according to the WHO classification) were graded in line with the Fédération Nationale des Centres de Lutte Contre le Cancer classification. Staging, where appropriate, was performed according to the tumour–node–metastasis (TNM) American Joint Committee on Cancer classification (Table 1).
Table 1. AJCC TNM and tumour grade staging system for sarcomaReference Edge, Byrd, Compton, Fritz, Greene and Trotti1

AJCC = American Joint Committee on Cancer; TNM = tumour–node–metastasis; G = tumour grade
The following variables were collated and analysed with regard to oncological outcome: age, gender, anatomical location, tumour size, histological type, metastasis, recurrence, surgical margins (R0 – no cancer cells at the resection margin, R1 – microscopic positive margin and R2 –macroscopic positive margin) and treatment modality.
Statistical analysis
The distributions of overall survival and disease-specific survival were calculated for each histological subtype using the Kaplan–Meier method. Overall survival was defined as the time in months from diagnosis to death from any cause. Disease-specific survival was defined as the time in months from diagnosis to death associated with the primary disease. Analyses were performed using a two-tailed Wilcoxon rank-sum test, wherein p = 0.05 (implemented in Matlab 7.3 software; Mathworks, Natick, Massachusetts, USA).
Results
Demographics
Sixty-eight adult patients (43 male, 25 female) were diagnosed with head and neck soft tissue sarcoma between January 1997 and December 2012. The median age at diagnosis was 53.5 years (range, 20–92 years).
Tumour types
Seventeen histological subtypes of sarcoma were identified. Angiosarcoma was the most frequently reported subtype within this series (n = 18), followed by the WHO ‘intermediate’ solitary fibrous tumour (n = 14). Two of the solitary fibrous tumours displayed histological features known to correlate with adverse outcomes, but were still categorised as intermediate tumours. Table 2 shows the breakdown of histological tumour subtypes.
Table 2. Histological subtypes based on WHO classificationReference O'Neill, Bilsky and Kraus7

* Two cases showed histological features known to correlate with adverse outcome.
† Two cases showed fibrosarcomatous dedifferentiation. WHO = World Health Organization
Presentation
All 18 angiosarcomas had a primary cutaneous origin in the face and/or scalp. A further 29 tumours arose in a cutaneous or subcutaneous location, whilst 21 arose in deep structures (e.g. 1 in the orbit, 1 from the hard palate, 2 from the nasal cavity and 2 from the ear canal). Tumours also arose from head and neck viscera, including two from the parotid gland, one from the submandibular gland, one involving the thyroid gland and tonsil, and two in the larynx.
At presentation, 60 patients (88.2 per cent) had a painless lump or lesion. Other symptoms included: haemoptysis from a laryngeal leiomyosarcoma, hoarseness from a laryngeal atypical lipomatous tumour, proptosis from a right orbit leiomyosarcoma, unilateral nasal obstruction from a nasal cavity leiomyosarcoma, shoulder and neck pain from a C3–C7 intradural leiomyosarcoma, and C5/C6 neuropathy from a malignant peripheral nerve sheath tumour.
Tumour size
Overall median tumour size, determined either on cross-sectional imaging or at the time of surgical excision in operative cases, was 25.0 mm (range, 5.5–135 mm). For angiosarcoma alone, this value was 34.0 mm; for the non-angiosarcomatous tumours, it was 25 mm.
Median overall survival for angiosarcoma cases was 17 months for patients with tumours less than 50 mm and 16.5 months for those with tumours more than 50 mm in size (p = 0.49). Median overall survival for all subtypes in which tumours were less than 50 mm and more than 50 mm was 23.5 months and 29 months, respectively (p = 0.30).
Stage at presentation
As TNM staging for soft tissue tumours is not appropriate for angiosarcomas, intermediate tumours, or those arising in an intracranial or visceral location, formal TNM staging has not been applied in this study.
Grading
Of the malignant tumour types identified, the Fédération Nationale des Centres de Lutte Contre le Cancer classification is applicable or predictive in only the following sarcoma subtypes: leiomyosarcoma (n = 8), undifferentiated sarcoma (n = 4), malignant peripheral nerve sheath tumour (n = 2) and synovial sarcoma (n = 1), a total of 15 cases.
On reviewing leiomyosarcoma (the most frequent histological subtype where the Fédération Nationale des Centres de Lutte Contre le Cancer classification is applicable), two patients had grade one disease, five had grade two disease and one had grade three disease. Three patients with grade two disease on histology died from the disease; one of these patients presented with distant metastasis and died after 4 months, one died from the disease after 2 months, and the other has not been cured but followed up for 116 months. The patient with a grade three leiomyosarcoma died after 96 months from the disease. The two patients with grade one disease were alive at last follow up (average of 48 months).
There were 53 head and neck soft tissue tumours where the Fédération Nationale des Centres de Lutte Contre le Cancer grading is not recommended by the WHO classification. These included the 18 angiosarcomas, the rare translocation sarcomas and the WHO intermediate forms of tumour (including those with ‘malignant’ features on histology).
Treatment
Overall, 54 patients underwent surgical intervention (Table 3). Complete resection was achieved in 37 patients (74 per cent). Fourteen out of 18 patients with an angiosarcoma underwent surgical intervention; complete resection was achieved in 8 cases (57 per cent). For the non-angiosarcomatous tumours, 29 out of 36 (80.5 per cent) were completely excised. The local control rate at five years in this series was 71.67 per cent.
Table 3. Patients treated with curative intent for each treatment modality

RT = radiotherapy; CRT = chemoradiotherapy
Nodal metastasis
Seven patients had nodal disease at initial presentation: one had a synovial sarcoma treated with surgery and adjuvant radiotherapy (RT), and one had an undifferentiated sarcoma treated with surgery and chemotherapy (doxorubicin and ifosfamide). There were five angiosarcoma patients with nodal disease: four had distant metastasis and were treated palliatively, and one was treated with surgery and axitinib. There was one leiomyosarcoma patient with nodal disease, again treated with surgery and adjuvant chemoradiotherapy (doxorubicin).
Distant metastasis
Eight patients presented with metastasis, all of whom were treated palliatively. Two patients declined further treatment, including five with angiosarcomas, one with leiomyosarcoma, one with undifferentiated sarcoma and one with a solitary fibrous tumour. Patients with metastasis at presentation had a median overall survival of 6 months, compared to 41 months in those without (p < 0.01).
Recurrence
Seventeen patients experienced recurrent disease, including 13 with WHO malignant types and 4 with WHO intermediate types. The WHO malignant types are described in Table 4. Notably, three patients with WHO intermediate type tumours suffered local recurrences (one dermatofibrosarcoma protuberans, one fibromatosis with nodal recurrence, and one solitary fibrous tumour).
Table 4. Recurrence and histological subtype

WHO = World Health Organization
Twelve patients with recurrence had complete resection (R0) following initial surgical excision. Complete resection (R0) was achieved in eight patients with an angiosarcoma; three of these tumours recurred locally and four did not recur. One patient was lost to follow up. Interestingly, two angiosarcomas with incomplete resection (R1) did not recur and were treated by surgery alone.
Survival
The disease-free survival rate at last follow up was 54.4 per cent. The median follow-up duration in our series was 18 months. Overall survival for this cohort of soft tissue sarcoma cases was 64 months, but this does not distinguish between intermediate and malignant subtypes.
Median overall survival was 26 months in patients aged below 30 years, and 17 months in those aged above 30 years (p = 0.31). Male to female ratio was 1.7:1. Median overall survival for males and females was 29 and 17 months, respectively (p = 0.63). The Kaplan–Meier survival curves for overall and disease-specific survival in Figure 1 show the different survival rates for intermediate histological subtypes and the malignant types. Angiosarcoma has the least favourable prognosis.
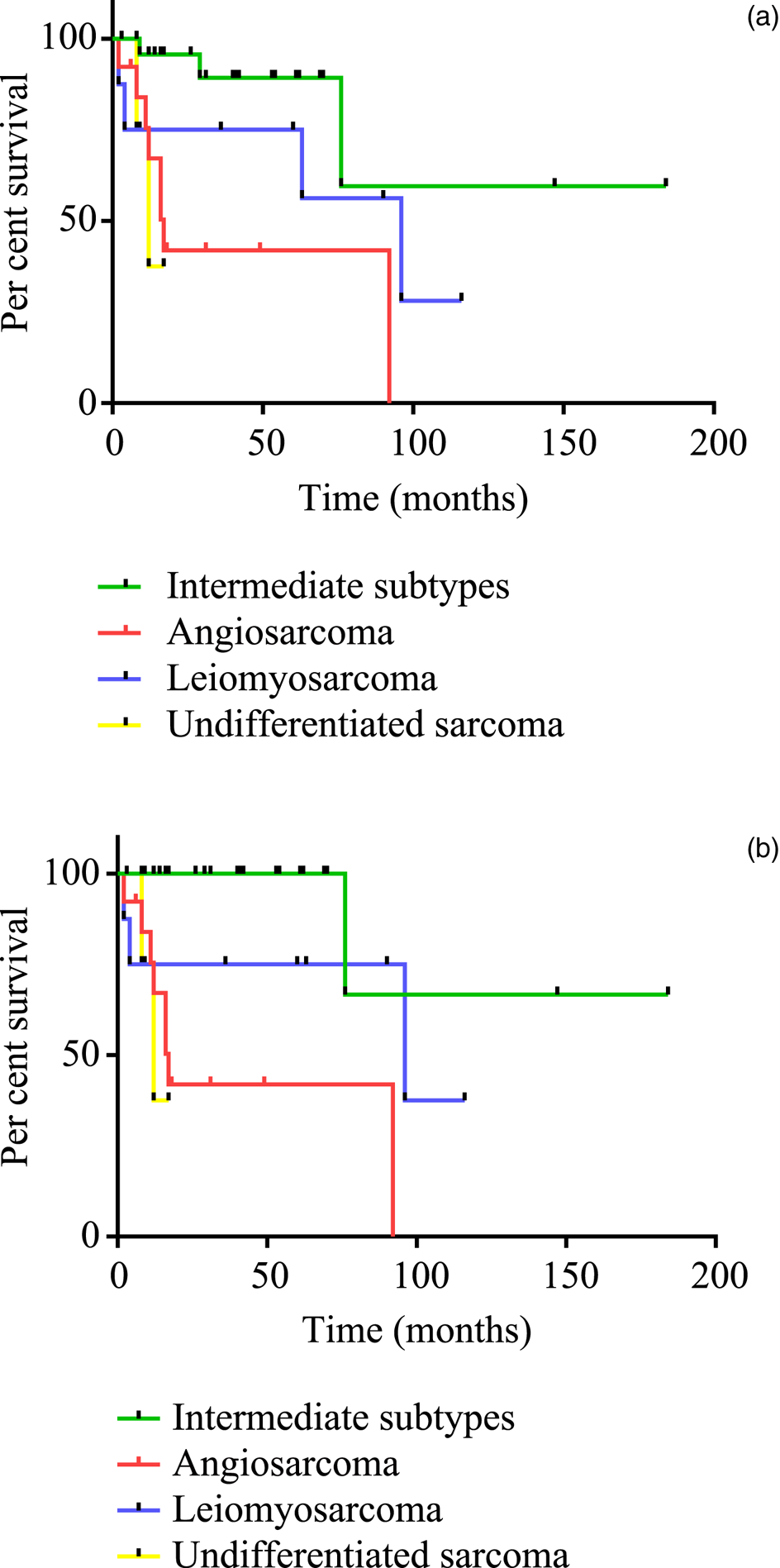
Fig. 1. Kaplan–Meier survival curves for (a) overall survival and (b) disease-specific survival, comparing World Health Organization intermediate and malignant tumour subtypes.Reference Fletcher, Bridge, Hogendoorn and Mertens4
Discussion
Head and neck soft tissue sarcoma accounts for approximately 2–15 per cent of all sarcomas, and represents approximately 1 per cent of head and neck malignancies.Reference Brockstein8 The number of each histological subtype varies between series; however, the most commonly represented are malignant fibrous histiocytoma,Reference Fletcher, Bridge, Hogendoorn and Mertens4 fibrosarcoma,Reference Fletcher, Bridge, Hogendoorn and Mertens4,Reference Potter and Sturgis9,Reference Bentz, Singh, Woodruff, Brennan, Shah and Kraus10 angiosarcoma, and malignant peripheral nerve sheath tumour as well as non-classified undifferentiated sarcoma.Reference Park, Roh, Kim, Cho, Choi and Nam11–Reference González-González, Bologna-Molina, Molina-Frechero and Domínguez-Malagon16 Both malignant fibrous histiocytoma and fibrosarcoma are diagnoses that have significantly decreased in incidence in recent years. This reduction in cases is largely because of the fact that most of these tumours can, with appropriate expertise and modern diagnostic methods, be assigned to a different category. In particular, fibrosarcoma is now a rare diagnosis, with many cases being found to be synovial sarcoma, malignant peripheral nerve sheath tumour or de-differentiated dermatofibrosarcoma protuberans.
Thus, it is not surprising that leiomyosarcoma is the most common non-angiosarcomatous malignancy in our series, reflecting modern sarcoma reporting frequencies. Rhabdomyosarcoma is also frequently reported as a common histological subtype; however, this does not feature in our series.Reference Park, Roh, Kim, Cho, Choi and Nam11 Angiosarcoma is the most frequently reported histological subtype in our series, with all cases presenting on the scalp or face, comparable to other series.Reference Park, Roh, Kim, Cho, Choi and Nam11 Within our study, angiosarcoma had the worse prognosis.
There were six rare malignant sarcomas (myxoid liposarcoma, epithelioid sarcoma, alveolar soft part sarcoma, synovical sarcoma, Ewing's sarcoma and extraskeletal myxoid chondrosarcoma) that presented as isolated cases, all of which are known to originate from a characteristic molecular abnormality, usually a balanced translocation.
Notably infrequent were the dedifferentiated liposarcomas, although five of their precursor lesions, atypical lipomatous tumours, were found. This is in keeping with the lack of deep adipose tissue in the head and neck, in contrast to the frequency of dedifferentiated liposarcoma at sites such as the retroperitoneum. There were 28 WHO intermediate type tumours, and the second most frequent diagnosis overall was solitary fibrous tumour (previously known as haemangiopericytoma) with 14 cases. These tumours behave in a way that is difficult to predict from histology.
The prognostic factors of head and neck soft tissue sarcoma remain unclear. In previous studies, sarcoma subtypes were grouped together for survival analysis, despite different biological behaviour.Reference O'Neill, Bilsky and Kraus7,Reference Bentz, Singh, Woodruff, Brennan, Shah and Kraus10 We have shown the varying prognoses between histological subtypes using the Kaplan–Meier method (Figure 1). In the literature, disease-specific survival rates range from 52 per cent to 60 per cent;Reference Kraus, Dubner, Harrison, Strong, Hajdu and Kher17,Reference Dijkstra, Balm, Coevorden, Gregor, Hart and Hilgers18 this is comparable to the rate of 54.4 per cent in our series.
We did not find age, gender or tumour size to be prognostic factors for survival, in comparison to other reviews in the literature.Reference Bentz, Singh, Woodruff, Brennan, Shah and Kraus10,Reference Willers, Hug, Spiro, Efird, Rosenberg and Wang19–Reference Cockerill, Daram, El-Nagger, Weber and Kupferman23 This may be because these factors do not reflect the behaviour of each histological subtype. However, our sample size is small and may limit the detection of such associations. Other studies have shown old age to be associated with significantly worse disease-specific survival; poorly differentiated grade and presence of nodal metastasis were also poor survival outcomes.Reference Park, Roh, Kim, Cho, Choi and Nam11,Reference Mark, Tran, Sercarz, Fu, Calcaterra and Juillard24 In the literature, salivary gland tumours have a worse prognosis as well, partly as a result of the high rates of recurrence.25 Comparatively, there were two patients with salivary gland soft tissue sarcomas: a fibrohistiocytic intermediate soft tissue giant cell tumour, which falls into the WHO intermediate classification, and an epithelioid haemangioendothelioma, which is a WHO malignant subtype, both of which were completely excised and did not recur.Reference Fletcher, Bridge, Hogendoorn and Mertens4,Reference Cockerill, Daram, El-Nagger, Weber and Kupferman23
Angiosarcoma has a poor prognosis.Reference Mark, Tran, Sercarz, Fu, Calcaterra and Juillard24 Five out of 18 patients (28 per cent) with angiosarcoma presented with distant metastasis. All angiosarcomas in our series presented as a lesion on the face or scalp. The extensive vascular supply to this area allows for rapid dissemination of the disease. In addition, angiosarcoma has a theoretical unique biological advantage compared to other tumours; every tissue to which malignant cells disperse is ‘natural’ (i.e. all vessels are lined by endothelium). Or, put another way, all tissues are fertile ‘soil’ for the ‘seed’ of metastatic angiosarcoma to establish itself. This is reflected in the histopathology of this disease where islands of angiosarcoma are often found on microscopy some distance from the main tumour.
Three out of eight cases with initial complete resection (R0) represented with recurrence, two of these with nodal recurrence. Two cases with incomplete resection did not recur. Thus, although negative margin (R0) clearance is desirable in the management of angiosarcoma, it does not necessarily correlate with survival in several studies.Reference Mark, Tran, Sercarz, Fu, Calcaterra and Juillard24,25 The achievement of negative margins through extensive resection needs to be balanced against post-operative functional outcome. Extensive resection in the head and neck can be associated with significant morbidity. Kraus et al., at the Memorial Sloan Kettering Cancer Center, advocate adjuvant RT for those with positive margins.Reference Kraus, Dubner, Harrison, Strong, Hajdu and Kher17
In patients with unresectable tumours or locally recurrent lesions, adjuvant RT should be considered. There may be a role for chemotherapy in the presence of distant metastasis, although improved outcomes are debatable.25 Furthermore, in those with metastatic soft tissue head and neck sarcoma, histology driven cytotoxic therapy is becoming the treatment paradigm: accurate histopathological diagnosis underpins oncological management as we move away from a ‘one size fits all’ approach.Reference Mark, Tran, Sercarz, Fu, Calcaterra and Juillard24
A surprising finding in our series is that the second most frequently presenting malignant or intermediate type soft tissue tumour in the head and neck region was solitary fibrous tumour. This lesion has suffered from a confusing nomenclature in the past and was previously referred to as haemangiopericytoma in many reports. This tumour is now known to harbour a specific cytogenetic abnormality, whereby the NAB2 and STAT6 genes on chromosome 12 are inverted and spliced together. All variants of this lesion have been shown to harbour this genetic abnormality. The mutation is associated with upregulation of the STAT6 gene, whose protein product can be reliably demonstrated immunohistochemically. This is a particularly challenging lesion to treat and follow up. Its behaviour is unpredictable based on the histological findings, and tumours with otherwise bland histology can recur or metastasise after many years. Thus, long-term follow up is currently recommended for these lesions, usually in conjunction with a sarcoma MDT team.
The importance of accurate pathological diagnosis is key in providing effective management for soft tissue head and neck sarcoma. Furthermore, pathological investigations are the basis for the accurate staging and stratification of clinical outcomes as stated by the UK National Multidisciplinary Guidelines.Reference Helliwell and Giles26
• Management of soft tissue head and neck cancer in 68 patients with varying histological subtypes was retrospectively reviewed
• Soft tissue head and neck sarcoma can arise in most head and neck regions, at any age, and be of any size
• Angiosarcoma was the most common subtype and has the least favourable prognosis
• Negative margins are desirable, but in soft tissue head and neck sarcoma such as angiosarcoma this may not correlate with improved survival
• The second most frequent diagnosis was solitary fibrous tumour (haemangiopericytoma), an intermediate type tumour
• Functional outcomes following extensive resection should be considered before surgery; this can affect quality of life, with no survival rate improvement
Our study is limited by sample size; however, it is the largest series of soft tissue head and neck sarcoma in the UK to date.Reference Singh, Grimer, Bhujel, Carter, Tillman and Abudu27 The rarity of certain histological subtypes mean that they may only present once in a decade; therefore, strong conclusions cannot be drawn from these data. Our analysis shows that distant metastasis is associated with significantly reduced overall survival. Complete resection at surgery is associated with a trend toward improved outcomes. Future studies, and meta-analyses of pooled data from multiple case series, will enable further evaluation of the evidence for surgical intervention in the treatment of these rare neoplasms.
Conclusion
Soft tissue head and neck sarcoma can arise in almost any region of the head and neck, at any age, and be of any size. Angiosarcoma is the commonest soft tissue malignancy in the head and neck, and it has a poor outcome in most cases. The second most frequent diagnosis in this series was solitary fibrous tumour (haemangiopericytoma), a lesion of intermediate behaviour. However, a large proportion of histological subtypes occur only once or twice in a decade, even within a large regional referral centre. This makes it challenging to draw conclusions on best management. An accumulation of evidence from relatively small case series will therefore be key in the long-term development of treatment strategies.
Histological subtypes should be treated on an individual basis given the clear differences in biological behaviour. At present, surgery is the mainstay of treatment for most soft tissue tumour types. Negative margins are desirable, but in soft tissue head and neck sarcoma such as angiosarcoma, this may not correlate with improved survival. Functional outcomes following extensive head and neck resections should be carefully considered before embarking on surgery; this may significantly impact on quality of life without an improvement in survival rates.
Competing interests
None declared







