Introduction
Clinicians are faced with an increasing assessment and management burden regarding patients presenting with acoustic neuroma.Reference Bakkouri, Romain, Guichard, Lot, Herman and Huy1,Reference Vernooij, Ikram, Tanghe, Vincent, Hofman and Krestin2 Many of these tumours may be small and asymptomatic, leading to management dilemmas, particularly given the potential for surgery or radiotherapy to cause deafness in the ipsilateral ear. The move towards ‘watchful waiting’ with serial imaging reduces treatment morbidity, but carries the risk of progressive hearing loss in addition to tumour growth.
Cochlear nerve function is usually considered to be significantly compromised by acoustic neuroma. Nevertheless, previous case studies have explored the use of cochlear implantation after acoustic neuroma removal following promontory stimulation to ensure an intact cochlear nerve.Reference Tran Ba Huy, Kania, Frachet, Poncet and Legac3,Reference Neff, Wiet, Lasak, Cohen, Pillsbury and Ramsden4 This can be effective, but carries the risk of progressive cochlear ossification in the weeks to months following surgery, which potentially renders successful implantation impossible. Other studies have described the successful technique of inserting a cochlear implant during the same operation as the removal of an acoustic neuroma from the ipsilateral ear.Reference Tran Ba Huy, Kania, Frachet, Poncet and Legac3–Reference Sanna, Sozzi, Medina, Prasad, Macak and Rossi8
This study aimed to confirm the feasibility of combining simultaneous cochlear implantation with resection of acoustic neuroma, and to develop a management algorithm for the reliable application of this technique. We hypothesised that, in selected cases, the protocol of ‘simultaneous cochlear implantation with resection of acoustic neuroma’ (termed ‘SCIRAN’ by the authors) would enable simultaneous tumour removal and cochlear implantation without increasing morbidity, thus permitting the rehabilitation of binaural hearing.
Materials and methods
Patient population
After obtaining approval from the Hunter Area Health Service Ethics Board, we conducted a retrospective review of prospectively collected data from consecutive patients undergoing simultaneous cochlear implantation with resection of acoustic neuroma at our centre between October 2012 and December 2015.
Indications for treatment
Patients were considered for simultaneous cochlear implantation with resection of acoustic neuroma based on the rationale that there was a realistic chance that adequate tumour removal for the clinical situation would not damage the cochlear nerve. Although the decision incorporated tumour size and location, strict criteria were not defined and the decision was instead based on the experience of the surgeon. Indeed, we do not consider there to be a maximum tumour size to preclude implantation, as the decision will be based on anatomical nerve integrity and the presence of electrically evoked auditory brainstem responses (ABRs) at the completion of tumour resection. Indications for inclusion are included in Table 1.
Table 1. Patient demographics and indications for surgery, with tumour size and location

*Maximal dimension of tumour. †Location determined based on pre-operative imaging. Pt no. = patient number; IAC = internal auditory canal; CPA = cerebellopontine angle; NF2 = neurofibromatosis type 2
Involvement of the patient in the treatment discussions was critical given the importance of patient commitment to rehabilitation, the residual hearing loss with tumour removal, and the possibility of cochlear nerve loss which would preclude implantation. Following discussion with the surgeon, each patient's primary concern was listed as the ‘indication’ for surgery, though in reality the decision to proceed was often multifactorial.
We included patients with both incidental and symptomatic tumours, and patients’ wishes were paramount in the final decision for surgery. Patients who could not undergo simultaneous cochlear implantation with resection of acoustic neuroma because of the lack of a functional cochlear nerve after tumour removal were excluded. All patients undergoing a combined procedure during the time period were included.
In Australia, patients need to meet very strict criteria to be eligible for a cochlear implant funded by the public health system, while those with private cover are able to access an implant without meeting such stringent criteria. In cases where we felt the patient would benefit from the procedure but they were not able to access the financial support for the implant, implant companies were approached to assist in donating the implant for research purposes.
Operative technique
All cases were performed by the senior author (RE), with the involvement of a second ENT surgeon (KK) in three cases and a single neurosurgeon (RF) in four cases. The surgical procedure involved a translabyrinthine approach, with facial nerve monitoring and electrically evoked ABR testing. The full operative protocol is outlined in Table 2. Illustrations of the operative field and electrode placement for intra-operative monitoring are provided in Figures 1 and 2.
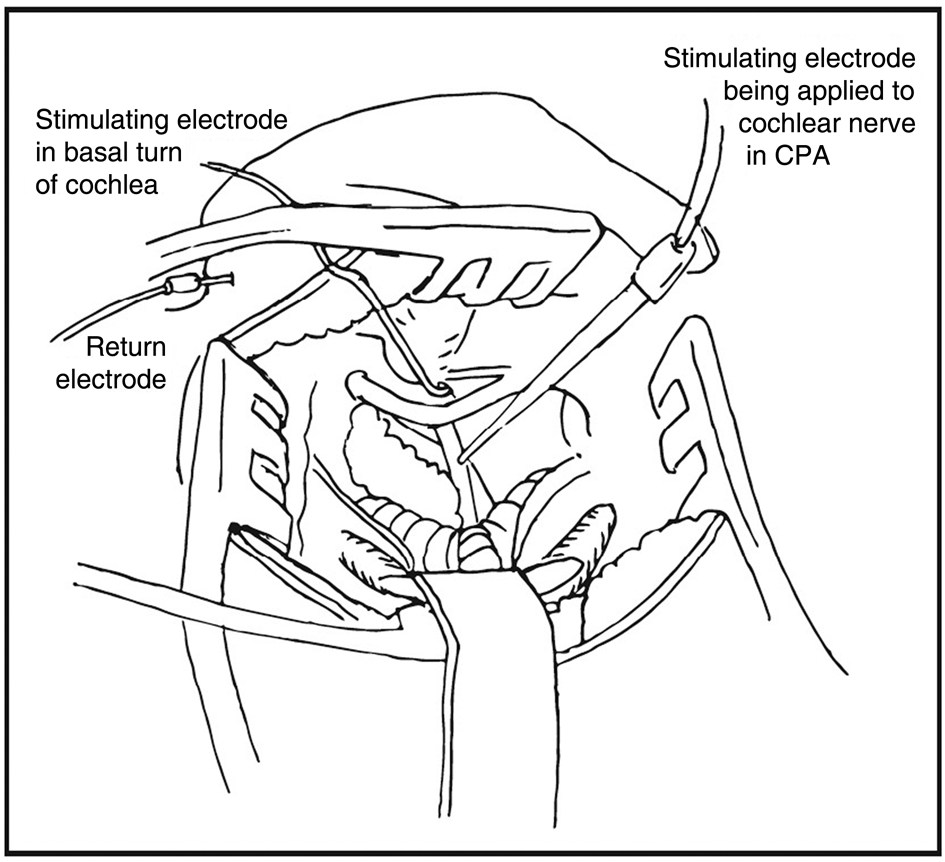
Fig. 1. View of operative field, with demonstration of monitoring technique. CPA = cerebellopontine angle

Fig. 2. Electrode placement for intra-operative monitoring. EABR = electrically evoked auditory brainstem response testing
Table 2. Protocol for simultaneous cochlear implantation with resection of acoustic neuroma

ABR = auditory brainstem response; IAC = internal auditory canal; CPA = cerebellopontine angle; IV = intravenous; CT = computed tomography; MRI = magnetic resonance imaging
The stimulating electrode for electrically evoked ABR testing was placed either in the round window niche or in the basal turn of the cochlea. The position of this electrode was checked repeatedly throughout the procedure, and particularly prior to any electrically evoked ABR testing. A separate stimulating electrode was used to help identify the cochlear nerve in the cerebellopontine angle.
Outcome measures
Any intra-operative or post-operative complications were recorded. Patients were reviewed immediately after surgery, and at three months and six months post-operatively. Post-operative hearing assessment was carried out by an audiologist according to Sydney Cochlear Implant Centre protocols, and included speech discrimination evaluation and scoring with the Category of Auditory Performance 7 system.Reference Archbold, Lutman and Nikolopoulos9 Tumour residual was assessed with either computed tomography (CT) or 1.5 Tesla magnetic resonance imaging (MRI) at six months’ follow up in all but one patient.
Results
A total of eight patients (five female, three male) underwent simultaneous cochlear implantation with resection of acoustic neuroma at our centre between October 2012 and December 2015. All patients had acoustic neuromas apart from one (patient number 2) who had an internal auditory canal meningioma. Patients’ ages ranged from 16 to 72 years.
Two patients underwent simultaneous cochlear implantation with resection of acoustic neuroma for bilateral profound hearing loss, two underwent the procedure primarily for tinnitus suppression and four chose to undergo the surgery for tumour resection (Table 1). Importantly, these indications represent the primary indication from the patients’ point of view following an informed discussion with the surgeon. In reality, the decision was multifactorial and included weighing up the risks of observing the tumour with those of removal.
There were no intra-operative complications. Patient number 8 underwent a subtotal excision, while all other patients underwent a total tumour excision. Follow-up duration ranged from 25 to 63 months. Two patients suffered from vertigo at the initial post-operative visit and one from tinnitus, though both of these symptoms had almost resolved at one month. One patient suffered facial nerve neuropraxia, which had fully resolved by one month.
Seven patients reported subjective benefits in their hearing following the procedure, and became continuous users of their implant (Table 3). Formal audiology results as available are displayed in Table 3; however, because of changes in post-operative management algorithms at our centre during the trial period and challenges in measuring speech discrimination in single-sided deafness cases, full audiological outcomes are not available.
Table 3. Hearing outcomes for our series of patients

*At 0.5, 1, 2, 4 kHz. †Direct input for single-sided deafness. Pt no. = patient number; PTA = pure tone audiometry; pre-op = pre-operative; CUNY = City University of New York Sentence Test; BKB = Bamford–Kowal–Bench Sentence Test; CNC = consonant–nucleus–consonant test; N/A = not available; CVC = consonant–vowel–consonant test
One patient received no aural percept from her implant, despite preserved intra-operative electrically evoked ABR potentials. She elected for implant removal at six months, and it was replaced with a bone-anchored hearing aid with good effect. One patient (with neurofibromatosis type 2 (NF2)) experienced gradual deterioration of his initially good auditory percept, and became a non-user of the cochlear implant over two years; this seemed to correlate with regrowth of the tumour residual after initial subtotal removal.
For patients who underwent simultaneous cochlear implantation with resection of acoustic neuroma because of their preference rather than a hearing indication, there were no improvements in their hearing post operatively. However, the tumour could be resected with hearing preservation in all patients who underwent surgery for this indication.
Discussion
The burden of acoustic neuroma is rising, with the tumour reported as being found in 0.2 per cent of asymptomatic patients undergoing a cerebral MRI.Reference Bakkouri, Romain, Guichard, Lot, Herman and Huy1,Reference Vernooij, Ikram, Tanghe, Vincent, Hofman and Krestin2 Most commonly, an acoustic neuroma is identified on MRI in a patient with asymmetrical sensorineural hearing loss. On occasion, an acoustic neuroma is identified during evaluation for cochlear implantation in a patient who is bilaterally deaf. During initial discussions regarding tumour treatment options, presenting complaints such as hearing loss are frequently set aside. Nevertheless, these benign tumours present a significant management challenge; if left untreated, many tumours will ultimately lead to deafness in the ipsilateral ear, while hearing can also be adversely affected by the various treatment modalities available.Reference Bakkouri, Romain, Guichard, Lot, Herman and Huy1 A particular challenge arises when the acoustic neuroma affects the only hearing ear.
In recent years, there has been a trend towards the conservative management of acoustic neuromas, particularly in patients with small asymptomatic tumours.Reference Smouha, Yoo, Mohr and Davis10 In a series of patients who underwent conservative management, between 50 and 58 per cent of tumours exhibited no growth, and 51 per cent did not affect hearing, though progression to definitive treatment occurred in 20–25 per cent of patients.Reference Smouha, Yoo, Mohr and Davis10 Factors predictive of rapid tumour growth have proved difficult to elucidate, and rapid tumour growth can occur at any stage.Reference Bakkouri, Romain, Guichard, Lot, Herman and Huy1,Reference Smouha, Yoo, Mohr and Davis10
Unfortunately, with these high rates of progression to definitive treatment, there is ensuing damage to the cochlea or cochlear nerve in the interim, and the loss of subsequent options to restore hearing.Reference Sughrue, Kane, Kaur, Barry, Rutkowski and Pitts11 High attrition rates have been reported in studies that evaluated conservative management, with hearing loss more likely to occur in patients who do not undergo regular review (as in patient 5).Reference Smouha, Yoo, Mohr and Davis10 When follow-up duration is long enough, many patients will show hearing loss in the ipsilateral ear, even with small tumours that do not grow.Reference Sughrue, Kane, Kaur, Barry, Rutkowski and Pitts11
The mechanisms of hearing loss in acoustic neuroma could include nerve compression or invasion, or impairment of the blood supply to the cochlear nerve or cochlea itself.Reference Sughrue, Kane, Kaur, Barry, Rutkowski and Pitts11,Reference Matsunaga and Kanzaki12 After long-term follow up in patients with small tumours of negligible growth, many of those affected will still lose hearing, and this phenomenon has been postulated to be caused by a subtle reduction in cochlear blood flow.Reference Sughrue, Kane, Kaur, Barry, Rutkowski and Pitts11 Faster growing tumours are known to impair hearing more than slow-growing tumours, and it is unclear whether this is due to compression of the cochlear blood supply at a more rapid rate than that at which collaterals develop or the compression of the nerve directly, or whether these represent a more aggressive subgroup of tumours invading the cochlear nerve.Reference Sughrue, Kane, Kaur, Barry, Rutkowski and Pitts11
Histological studies have revealed that the degree of auditory and vestibular dysfunction produced is independent of the number of nerve fibres destroyed, and thus ‘conduction block’ and cochlear functional changes have been implicated.Reference Matsunaga and Kanzaki12 Examination of chronically compressed cochlear nerves reveals abnormalities in myelin sheaths and preservation of micro-vessels, but with hypertrophy and hyperplasia consistent with mild to moderate reduction of endoneurial blood flow.Reference Matsunaga and Kanzaki12 The reduction in blood flow may contribute to VIIIth ‘nerve block’ and, as such, there is potential for hearing improvement after removal of the pressure imposed by an acoustic neuroma.Reference Matsunaga and Kanzaki12 Occlusion of the internal auditory artery or its branches within the internal auditory canal is another proposed mechanism, potentially causing ischaemic damage to the cochlear nerve or the cochlea.Reference Badie, Pyle, Nguyen and Hadar13 Histological studies from the cochlea reveal significant levels of inner and outer hair cell loss, cochlear neuronal loss, and increased endolymph and perilymph precipitate when compared to the opposite ear.Reference Roosli, Linthicum, Cureoglu and Merchant14 Lower internal auditory canal pressures have been correlated with better pre-operative hearing levels, supporting the concept of early intervention.Reference Badie, Pyle, Nguyen and Hadar13
Results from studies of hearing preservation surgery outcomes may guide us in terms of patient selection for simultaneous cochlear implantation with resection of acoustic neuroma. Hearing outcomes rather than cochlear nerve function are primarily reported in the hearing preservation surgery literature, and vary widely between 2 and 93 per cent, generally with higher rates in more recent reports.Reference Kari and Friedman15 Favourable results have been demonstrated in cases of large tumours, tumours with intracanalicular extension, tumours with prolonged physical association with the cochlear nerve, and more medial tumours.Reference Yong, Westerberg, Dong and Akagami16–Reference Rowed and Nedzelski19 Tumour recurrence rates following nerve-conserving surgery are improving, reaching as low as 0.5–0.7 per cent in recent reports.Reference Samii, Gerganov and Samii20
Mechanisms of intra-operative hearing loss are labyrinthine, neural and vascular.Reference Colletti and Fiorino21 If a clear plane between the tumour capsule and the facial or cochlear nerve is identified, this correlates with a better chance of preserving the nerves. If, however, there are extensive adhesions, the cleavage plane should be developed in the vestibular nerve–tumour interface or the capsule–tumour interface, to optimise the chances of retaining nerve function, as this represents the ‘sub-perineural’ space.Reference Sasaki, Shono, Hashiguchi, Yoshida and Suzuki22 Meticulous efforts to reduce pressures in the internal auditory canal may lead to better hearing outcomes, including sharp proximal to distal dissection of cranial nerves, careful drilling in the internal auditory canal and minimisation of nerve manipulation.Reference Badie, Pyle, Nguyen and Hadar13,Reference Colletti and Fiorino21
As early as 17 hours after translabyrinthine surgery, there is diffuse haemorrhage in all cochlear partitions. After two weeks, the haemorrhages are less manifest, but hyalinisation is seen within the spiral ligament. Specimens examined from 4 to 11 years exhibit total cochlear ossification, with near-total loss of spiral ganglion cells.Reference Belal23 Despite these findings, some spiral ganglion cells survive for years after damage to the otic capsule, and patients will continue to respond to electrical stimulation by an intra-cochlear electrode. Cochlear implantation can be effective following this approach, but should be undertaken as quickly as possible to minimise the risk of cochlear fibrosis and ossification, which will take place in the short to medium term.Reference Belal23
Facial nerve monitoring is well established in acoustic neuroma resection and provides real time information to the surgeon. It has been shown to correlate with excellent facial nerve outcomes following surgery.Reference Oh, Nagasawa, Fong, Trang, Gopen and Parsa24 Advances in cochlear nerve monitoring yield significant hope of improving cochlear nerve outcomes as well.
The first technology used for cochlear nerve monitoring was brainstem auditory evoked potentials; this improved outcomes, but had the disadvantages of time delay, and false positives related to anaesthesia, hypothermia or irrigation.Reference Oh, Nagasawa, Fong, Trang, Gopen and Parsa24 Electrocochleography is a ‘near field’ technique that relies on electrode placement on the promontory, and has been used with success in some centres.Reference Oh, Nagasawa, Fong, Trang, Gopen and Parsa24 The major drawback is insensitivity to changes in the proximal portion of the nerve, and persistence of electrocochleography action potentials has been documented 25 minutes after cochlear nerve section.Reference Colletti and Fiorino21 Cochlear nerve action potentials are a newer technology used in some units and represent a ‘near field’ measurement of cochlear nerve function.Reference Oh, Nagasawa, Fong, Trang, Gopen and Parsa24 Thus, there are significantly shorter latency periods with more ‘real time’ information available, which has been shown to correlate with post-operative hearing outcomes.Reference Oh, Nagasawa, Fong, Trang, Gopen and Parsa24 This technique has the technical disadvantage of difficulties in finding space to place the recording electrode in large tumours. Electrically evoked ABRs have not been extensively studied in acoustic neuroma surgery, but have been routinely used for many years by our group (at the Sydney Cochlear Implant Centre) using the cochlear implant itself at completion of (non-tumour) implantation surgery.Reference Kari and Friedman15,Reference Walton, Gibson, Sanli and Prelog25
Overall, no technology has proved completely effective in ensuring the integrity of the cochlear nerve intra-operatively, and this area is in need of further research. Our current protocol is to perform intra-operative cochlear nerve monitoring in all tumour surgical procedures to examine the integrity of the cochlear nerve pre- and post-tumour resection. Only patients with retained electrically evoked ABRs are candidates for immediate implantation.
Other technologies used for hearing rehabilitation in acoustic neuroma patients include bone conduction implants and auditory brainstem implants. Bone conduction implants have been used in patients with single-sided deafness, with positive effects on speech reception and significant subjective benefit.Reference Yuen, Bodmer, Smilsky, Nedzelski and Chen26 Problems include pain, headache, skin irritation or infection, and background noise amplification.Reference Yuen, Bodmer, Smilsky, Nedzelski and Chen26 Sound lateralisation is present, but localisation is poor.Reference Hol, Bosman, Snik, Mylanus and Cremers27 Auditory brainstem implants have been used in patients in whom cochlear nerve function has not been preserved. In this situation, the hearing outcomes are not as good as a cochlear implant in a patient with single-sided deafness, nor one with an acoustic neuroma in the only hearing ear; nevertheless, it represents a reserve option if simultaneous cochlear implantation with resection of acoustic neuroma is not possible.Reference Tran Ba Huy, Kania, Frachet, Poncet and Legac3,Reference Aristegui and Denia5
Cochlear implantation in patients with single-sided deafness may improve: sound and speech perception, the likelihood of developing or regaining auditory and verbal language abilities, tinnitus, and educational outcomes.Reference Bichey and Miyamoto28 Binaural hearing increases the ability to localise sound and interpret speech in the face of background noise, and has been shown to improve quality of life indicators. Several studies have demonstrated improvements in speech understanding and localisation, tinnitus reduction, and enhanced quality of life through employing cochlear implantation to gain binaural hearing, and large studies are currently underway to evaluate this treatment option further.Reference Bichey and Miyamoto28–Reference Kitterick, O'Donoghue, Edmondson-Jones, Marshall, Jeffs and Craddock30 Bilateral cochlear implantation has been shown to be cost effective when compared to many other medical procedures.Reference Bichey and Miyamoto28
Cochlear implantation following acoustic neuroma surgery has been documented in multiple case reports and series, with reasonable success reported.Reference Tran Ba Huy, Kania, Frachet, Poncet and Legac3,Reference Neff, Wiet, Lasak, Cohen, Pillsbury and Ramsden4 In most of these cases, the authors made use of promontory stimulation in the weeks post-operatively, to ensure that cochlear nerve function remained at a level conducive to cochlear implant insertion. Interestingly, in some cases where promontory stimulation was initially negative, the test repeated at a later date became positive, potentially due to the resolution of intra-operative bleeding and post-operative oedema.Reference Neff, Wiet, Lasak, Cohen, Pillsbury and Ramsden4 As such, the test should be repeated at six to eight weeks, as cochlear implantation may still be possible even when cochlear nerve function appears impaired during the operation.Reference Neff, Wiet, Lasak, Cohen, Pillsbury and Ramsden4 While there has been some concern regarding the long-term loss of cochlear implant function because of cochlear nerve scarring over time, the results of long-term follow up of over five years have been promising.Reference Neff, Wiet, Lasak, Cohen, Pillsbury and Ramsden4 Where there is doubt as to the functionality of the cochlear nerve, an option is to insert a sleeper array until promontory stimulation is performed. Other authors have shown this to be an effective method.Reference Hassepass, Arndt, Aschendorff, Laszig and Wesarg31
• Neuro-otologists face an increasing burden of acoustic neuroma
• Cochlear implants have expanded management options; they have been used in delayed or simultaneous procedures with tumour resection
• By combining the procedures, the cochlear ossification risk precluding implantation is removed
• Advances in neuro-monitoring allow intra-operative confirmation of cochlear nerve integrity
• A large cohort of patients undergoing simultaneous cochlear implantation with acoustic neuroma resection is described
• This procedure was safe and effective in maintaining or restoring hearing, thus expanding the neuro-otologist's options
There are 37 previously published cases of simultaneous cochlear implantation and acoustic neuroma removal at the time of writing (reported within 10 studies).Reference Tran Ba Huy, Kania, Frachet, Poncet and Legac3–Reference Sanna, Sozzi, Medina, Prasad, Macak and Rossi8,Reference Belal23,Reference Hulka, Bernard and Pillsbury32–Reference Rooth, Dillon and Brown34 None of the papers reported complications, and all but one documented good hearing outcomes post-operatively. One report mentioned the intra-operative assessment of cochlear nerve function, which was achieved with intra-operative telemetry through the implant, as was utilised in our first patient. In a small series, the cochlear nerve was monitored with a golf club electrode at the round window niche.Reference Lloyd, Glynn, Rutherford, King, Mawman and O'Driscoll33 We also utilised electrically evoked ABR testing in our patients, with success; this is a cheaper option than monitoring through the implant if the cochlear nerve proves non-functional. We were unable to identify any reports of patients undergoing cochlear implantation during the same operation as the removal of a cerebellopontine angle meningioma, making patient 2 the first reported case of this operation, and highlighting that this algorithm is possible not only in acoustic neuroma but in other cerebellopontine angle tumour resections. Many authors did not monitor the cochlear nerve and apparently made the decision to implant based on anatomical assessment of nerve integrity.
Other authors have examined the use of a cochlear implant in the ear affected by the tumour, or the employment of contralateral implantation prior to tumour removal.Reference Lella, Merkus, Trapani, Taibah, Guida and Sanna35–Reference Carlson, Neff, Sladen, Link and Driscoll38 These options highlight the added complexity of considering the use of cochlear implants in the management of acoustic neuroma, thus emphasising the importance of an individualised treatment plan for each patient.
There is increasing evidence that 1.0–1.5 Tesla MRI scanning is safe in patients with a cochlear implant, and that it reveals useful diagnostic information.Reference Crane, Gottschalk, Kraut, Aygun and Niparko39,Reference Baumgartner, Youssefzadeh, Hamzavi, Czerny and Gstoettner40 New technologies in cochlear implants such as ‘self-aligning’ magnets mean that they are increasingly safer in MRI, and stronger magnets with better image resolution are becoming safe.Reference Baumgartner, Youssefzadeh, Hamzavi, Czerny and Gstoettner40 Clinically significant recurrence (i.e. brainstem compression) would be clearly visible on high-resolution CT, although this is not the ‘gold standard’ method of surveillance following surgery.
Acoustic neuroma management in patients with NF2 presents significant challenges, and the debate about early versus late resection is ongoing.Reference Neff, Wiet, Lasak, Cohen, Pillsbury and Ramsden4 Acoustic neuromas in these patients grow rapidly, and without intervention hearing loss is almost universal.Reference Neff, Wiet, Lasak, Cohen, Pillsbury and Ramsden4,Reference Aristegui and Denia5 As exhibited by patient 8 in our series, the possibility of simultaneous cochlear implantation with resection of acoustic neuroma represents a potential option in the management of these patients. This was a large tumour at presentation, necessitating incomplete tumour removal in order to preserve facial and cochlear nerve function, probably dooming the patient to recurrence and loss of the cochlear implant. We feel the option of early tumour removal in order to provide a better chance of retaining hearing, made possible by the simultaneous cochlear implantation with resection of acoustic neuroma protocol, still warrants further investigation in patients with this disease.
Conclusion
Simultaneous cochlear implantation with resection of acoustic neuroma represents a new approach for the neuro-otologist facing the growing burden of acoustic neuroma. The simultaneous cochlear implantation with resection of acoustic neuroma algorithm allows the individualisation of treatment plans according to both patient and tumour factors. It appears safe, and we saw no additional morbidity in our series. The combination of these established procedures allows the surgeon to overcome the risk of progressive cochlear ossification, which will inevitably occur in the short term following translabyrinthine surgery. Intra-operative electrically evoked ABR testing can be used to monitor the cochlear nerve before, during and after tumour resection. If there is doubt as to the function of the nerve, an inexpensive ‘sleeper array’ can be implanted while waiting for promontory stimulation following surgery.
The potential applications are large and will continue to expand, particularly as the indications for the management of single-sided deafness continue to grow. Future research is required in order to maximise the reliability of the intra-operative cochlear nerve function assessment, and to further assess the outcomes of simultaneous cochlear implantation with resection of acoustic neuroma by identifying the patient and disease factors that predict beneficial surgery.
Acknowledgements
The authors would like to thank: MedEl and Cochlear™ Australia, for their kind donation of a cochlear implant for two of our patients who did not meet the current Australian requirements for public funding of a Cochlear implant; Carmel Ramsey (Sydney Cochlear Implant Centre), for kindly providing audiological assessment of the study patients; and A/Prof Gary Hoffman and Dr Tom Kertesz, for providing advice regarding production of the manuscript.
Competing interests
None declared







