Introduction
Of all the early post-operative complications of total laryngectomy, pharyngocutaneous fistula formation is the commonest. Its incidence ranges from 3 to 65 per cent,Reference Galli, De Corso, Volante, Almadori and Paludetti 1 and it reduces the quality of life of patients by delaying oral feeding and prolonging hospitalisation. If not treated satisfactorily, pharyngocutaneous fistula can lead to fatal complications such as sepsis and carotid artery rupture.
The aetiology of post-operative pharyngocutaneous fistula formation is multifactorial, and there is still controversy over how to identify high-risk patients. Many risk factors have been investigated in individual reports and meta-analyses;Reference Paydarfar and Birkmeyer 2 however, there is still disagreement on which factors predispose to pharyngocutaneous fistula formation. Increasing importance is being ascribed to factors such as pre-operative radiotherapy,Reference Tsou, Hua, Lin, Tseng, Tsai and Shaha 3 tumour size and systemic factors (e.g. diabetes, cardiovascular disease, and post-operative haemoglobin levels lower than 12.5 g/dl).Reference Boscolo-Rizzo, De Cillis, Marchiori, Carpenè and Da Mosto 4 All of these factors lead to capillary network damage. Consequently, the pharyngeal mucosa remaining after surgery is friable and poorly vascularised; moreover, tissue in the field of radiation treatment has an altered extracellular matrix, with areas of fibro-atrophy. This combination of poor vascularisation and tissue traction does not promote good healing, and facilitates pharyngocutaneous fistula formation.Reference Bohannon, Carroll, Magnuson and Rosenthal 5
The purpose of this study was to examine the effectiveness of the Montgomery salivary stent in the prevention of pharyngocutaneous fistula formation in very selected cases with a high risk of suture dehiscence after pharyngolaryngectomy. The Montgomery salivary stent device is affordable, easy to apply and well tolerated by patients. It is generally used for pharyngocutaneous fistula treatment but not, as yet, for pharyngocutaneous fistula prevention.
Materials and methods
Between February 2002 and December 2011, we identified a retrospective series of patients who had undergone surgical resection of laryngeal-hypopharyngeal tumours at the otolaryngology and head and neck surgery department of S Raffaele Hospital, Milan. We analysed cases undergoing extended laryngectomy with bilateral neck dissection, and those undergoing partial or complete pharyngolaryngectomy with bilateral neck dissection.
Of the 107 cases examined, the histological diagnosis was squamous cell carcinoma in 104 patients, chondrosarcoma in 2 patients and thyroid carcinoma extending to the larynx in 1 patient. Neoplasms were staged according to the International Union Against Cancer tumour-node-metastasis system, as follows: 45.8 per cent as T4, 44 per cent as T3 and 10.2 per cent as T2.
The study compared two groups. The first group was composed of 31 patients, extracted from our series of 85 patients surgically treated between 2002 and 2008 and ranging in age from 40 to 85 years. These patients were at increased risk of fistula development due to the extent of their tumour (i.e. extension to the base of the tongue, cervical skin or pyriform sinuses) or due to prior radiation therapy (35 per cent of these patients had received radiation therapy between 6 and 18 months before surgery).
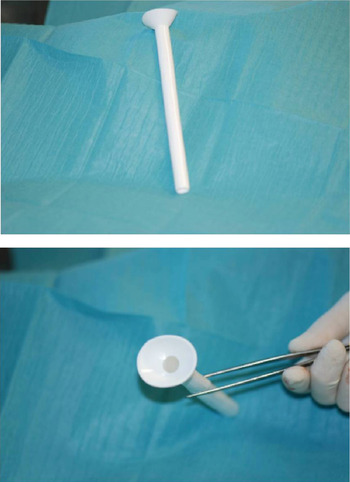
The second group was composed of 22 patients who underwent the same kind of surgery between 2009 and 2011. Of these patients, 72.7 per cent were already in stage IV, and 63.6 per cent had been treated with radiochemotherapy. In this group of 22 patients, a Montgomery salivary stent was placed in advance during reconstruction of the neopharynx. A radiopaque model was used with a diameter ranging from 16 to 20 mm (Figure 1).
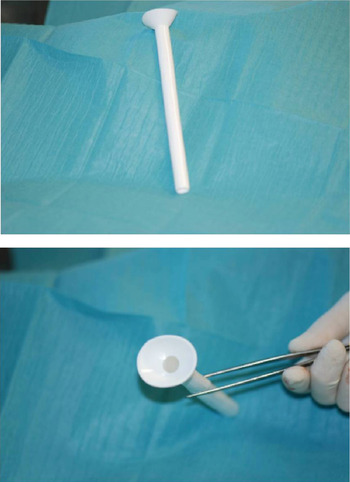
Fig. 1 Montgomery salivary stent, radiopaque model.
After a mean ± standard deviation (SD) of 35 ± 10 days post-operatively, the stent was removed transorally with a haemostat under local anaesthesia, as the superior lip can usually be palpated at the level of the posterior tongue. In the absence of post-operative complications, patients were discharged on the 15th post-operative day on average, and were subsequently re-hospitalised after a mean ± SD of 20 ± 10 days for salivary stent removal and radiological monitoring. In all patients, surgical procedures were performed observing general rules of sterility. Neopharynx reconstruction was performed in accordance with presurgical planning, including a direct, T-shaped suture in two layers with adsorbent material in cases that did not require ab initio reconstruction using a flap. Transposition of a pedicle or free flap (pectoralis major or radial forearm) or a gastric ‘pull-up’ was used after partial or complete excision of the hypopharynx.
Statistical comparison of the results from the two subgroups was performed using the chi-square test, or Fisher's exact test for small sample sizes. A p value of less than 0.01 was considered statistically significant.
This study conformed to the ethical standards of the last version of the Declaration of Helsinki (published in 1964).
Results
In the first group, 31 patients undergoing surgery between 2002 and 2008, only 10 patients underwent neopharynx reconstruction with a pedicle or free flap, while in the other 21 a direct, T-shaped suture was sufficient. After primary surgery, 14 patients (45 per cent) developed a pharyngocutaneous fistula. Nine of these 14 patients (64 per cent) achieved spontaneous closure with conservative treatment including: systemic antibiotic therapy (amoxicillin plus clavulanate 1.2 g intravenously, thrice daily); daily topical medication and removal of necrotic tissue; local cleaning with antibiotic solution; and application of a compressive dressing. This therapeutic choice was associated with a significant increase in the duration of hospitalisation (p = 0.01) (this group's mean ± SD hospitalisation duration was 35 ± 10 days). The other 5 of the 14 patients (36 per cent) did not achieve spontaneous recovery within 1 month and required revision surgery with cosmetic reconstruction.
In the second group, 22 patients undergoing surgery between 2009 and 2011, 6 required a pectoralis major flap and 5 a radial forearm free flap for neopharynx reconstruction. Two patients needed a pectoralis major flap for pre-laryngeal skin reconstruction. In all 22 cases, a Montgomery salivary stent was used. Only 2 of these 22 cases (9 per cent) developed a post-operative fistula; one was finally resolved by transposition of a pedicled myocutaneous flap of pectoralis major muscle, and the other with conservative treatment.
Statistical analysis showed that the application of the salivary stent significantly reduced the incidence of pharyngocutaneous fistula (p < 0.01). In addition, there was no statistically significant difference between the two groups in terms of primary closure of the neopharynx (with a direct T suture or flap) and development of fistula.
Discussion
Pharyngocutaneous fistula is a common complication after total pharyngolaryngectomy. It is generally considered to be a serious problem for the patient because of the delay in restoration of oral feeding, prolonged hospitalisation, and need for revision surgery.
The aetiology of pharyngocutaneous fistula is still debated. Several aetiological factors have been proposed and analysed in the literature, including: prior radiation therapy; location and size of the tumour; extent and methods of surgery; radical neck dissection; presence of residual tumour after surgery; poor general condition; and post-operative levels of albumin and haemoglobin.Reference Redaelli, Zinis, Ferrari, Tomenzoli, Premoli, Parrinello and Nicolai 6
As reported in other publications,Reference Tsou, Hua, Lin, Tseng, Tsai and Shaha 3 , Reference Virtaniemi, Kumpulainen, Hirvikoski, Johansson and Kosma 7 we believe that a patient's risk of developing a pharyngocutaneous fistula is increased by pre-operative radiation therapy and radiochemotherapy, and by extension of disease to the pyriform sinus and the base of the tongue. The development of radiochemotherapy organ preservation programmes has revolutionised the treatment of patients with advanced-stage laryngeal cancer, because of the high rate of larynx preservation. Hence, in contrast with the past, there is now a lower number of primary total laryngectomies and a growing number of salvage total laryngectomies after failure of radiochemotherapy, and this latter population of patients shows an increased incidence and severity of pharyngocutaneous fistula.Reference Ganly, Patel, Matsuo, Singh, Kraus and Boyle 8 , Reference Weber, Berkey, Forastiere, Cooper, Maor and Goepfert 9
Total laryngectomy remains the first choice only when extensive cartilage invasion, extralaryngeal involvement of soft tissue or poor laryngeal function (incompetent larynx) are present, 10 as functional recovery after primary radiochemotherapy is unlikely.
Faced with the results of our 2002–2008 patient series, and the growing number of fistulas due to more patients undergoing salvage surgery after radiotherapy, we decided to introduce the systematic usage of the Montgomery salivary stent. Our inclusion criteria for stent placement included: laryngectomy extending to the base of the tongue, hypopharynx, cervical oesophagus or cervical skin; and/or primary radiochemotherapy. The Montgomery salivary stent is placed during reconstruction of the neopharynx. The main indications for this stent are: pharyngocutaneous fistula after total laryngectomy; cervical oesophageal stenosis; and tracheoesophageal fistula.Reference Montgomery and Montgomery 11 , Reference Sevilla Garcia, Suarez Fente, Rodrigo Tapia and Llorente Pendas 12 There are two models available – transparent or radiopaque – with a diameter ranging between 8 and 20 mm. We preferred to use the radiopaque model with a diameter of 16–20 mm (larger diameter), as this model: (1) gives good protection of the neopharynx from saliva; (2) prevents stenotic scarring of the neopharynx (often the cause of fistula development); and (3) can be detected radiologically in the case of intestinal migration.Reference Bitter, Pantel, Dittmar, Guntinas-Lichius and Wittekindt 13 We found that the transparent model was better in the case of free flap reconstruction because it enabled flap survival to be monitored endoscopically. The stent material is extremely porous and loses its transparent nature every time a contrast medium such as methylene blue is used.
Our study compared two groups of patients undergoing pharyngolaryngectomy who had a high risk of fistula formation. The incidence of fistula formation was 45 per cent (14/31) in the first group but only 9 per cent (2/22) in the second group (which had the stent placed in advance); this difference was statistically significant (p < 0.01). Furthermore, there was no statistically significant difference between the two groups regarding either primary closure of the neopharynx (either with direct T suture or flap) or fistula development.
-
• Pharyngocutaneous fistula is a common complication of total laryngectomy
-
• Tumour extent and pre-operative radiochemotherapy are predisposing factors
-
• This study compared patients with these risk factors, treated with and without a preventative Montgomery salivary stent
-
• Stent placement significantly reduced fistula incidence
In addition, some authorsReference Gil, Gupta, Kummer, Cordeiro, Kraus and Shah 14 have shown that the procedure of buttressing the neopharyngeal suture line after total laryngectomy, using well vascularised, non-irradiated tissue (such as pectoralis major muscle), does not reduce the incidence of fistulas but only the time to spontaneous closure.
Conclusion
Our study has shown the importance of preventative application of a salivary stent in selected patients at high risk of fistula development. Used in this way, this stent is affordable, easy to apply and well tolerated by the patient. Furthermore, it prevents stenotic scarring of the neopharynx, and protects the suture lines from potential damage induced by contact with saliva, reducing post-operative morbidity and therefore the time and cost of hospitalisation.