Introduction
The diagnosis and management of recurrent cholesteatoma can be problematic for the otolaryngologist. The detection of recurrent cholesteatoma is important for treatment planning in these patients. Primary surgery can distort the anatomy, particularly after an intact canal wall mastoidectomy technique, which makes the clinical diagnosis of recurrent cholesteatoma difficult.Reference Liu, Chen, Wang and Huang1–Reference Lingam, Kumar and Vaidhyanath4
Different imaging modalities are used to assess cholesteatoma after surgery, as imaging can decrease the need for second-look (or re-look) surgery for detecting residual or recurrent disease.Reference Khemani, Singh, Lingam and Kalan5,Reference Razek, Ghonim and Ashraf6 Non-contrast magnetic resonance imaging (MRI) and high-resolution computed tomography (CT) are unreliable, and are limited in their ability to distinguish recurrent or residual cholesteatoma from cholesterol granuloma and granulation tissue.Reference Gamaleldin, Elsebaie, Khalifa, Abdel Razek, Mehanna and Shehata7–Reference Fukuda, Morita, Harada, Fujiwara, Hoshino and Nakamaru11
Diffusion-weighted imaging allows the discrimination of different tissues according to their physiological processes, because it represents the random motion of water protons, which is disturbed by intracellular organelles and macromolecules of the tissues.Reference Abdel Razek and Nada12,Reference Abdel Razek and Kamal13 Diffusion-weighted imaging is used for the evaluation of head and neck cancer pre- and post-therapy,Reference Abdel Razek and Nada12–Reference Abdel Razek, Mossad and Ghonim16 and has a role in the examination of external ear diseases.Reference Cherko, Nash, Singh and Lingam17,Reference Razek18
Few studies have discussed the role of diffusion-weighted imaging for the differentiation of recurrent cholesteatoma from granulation tissue.Reference Bazzi, Wong, Jufas and Patel19–Reference Clarke, Mistry, AlThubaiti, Khan, Morris and Bance27 This paper describes the use of diffusion-weighted imaging and delayed contrast MRI in the evaluation of recurrent cholesteatoma. This study aimed to assess the reliability of diffusion-weighted imaging in differentiating recurrent cholesteatoma from granulation tissue after intact canal wall mastoidectomy.
Materials and methods
Patients
This study was approved by the institutional review board and informed consent was obtained from the patients. This prospective study was conducted on 58 consecutive patients after intact canal wall mastoidectomy. Inclusion criteria were: patients with suspected recurrent or residual cholesteatoma after intact canal wall mastoidectomy. Two patients were excluded from the study because of motion artefacts.
The data for 56 patients, aged 16–45 years (mean of 26.8 ± 14.5 years), were analysed. These patients presented with otorrhoea (n = 43) and hearing loss (n = 33). All patients underwent diffusion-weighted imaging and delayed contrast MRI of the petrous bone. Final diagnoses were made based upon revision or second-look surgery.
Magnetic resonance imaging
Imaging was acquired using a 1.5 Tesla MRI machine (Achieva; Philips, Best, Netherlands) using an eight-channel head coil. T2-weighted turbo spin-echo images (repetition time = 1250 ms, echo time = 200 ms) and T1-weighted turbo spin-echo images (repetition time = 450 ms, echo time = 15 ms), in the axial plane, were obtained. Non-echo-planar spin-echo diffusion-weighted imaging was carried out, in the axial plane, using the following parameters: b values of 0, 500 and 1000 second/mm2; field of view = 23 cm and 23 cm; echo-planar imaging factor = 60; matrix acquisition = 112 × 89; slice thickness = 5 mm; inter-slice gap = 1 mm; repetition time = 1000 ms; and echo time = 110 ms. Axial, post-contrast T1-weighted images were obtained 40 minutes after the intravenous injection of 10 ml gadopentetate (gadolinium).
Image analysis
Image analysis was performed by one radiologist (Dr Abdel Razek; 25 years’ experience in head and neck imaging), who was blinded to the patients’ clinical presentations and the surgical findings. Restricted diffusion with high signal intensity of a lesion on diffusion-weighted imaging was interpreted as recurrent cholesteatoma (Figure 1). The largest diameter of the cholesteatoma was measured on diffusion-weighted imaging. The images were evaluated for delayed contrast enhancement. If the lesion was enhanced, it was interpreted as granulation tissue; a non-enhanced lesion was interpreted as recurrent cholesteatoma. A second reading was performed by the same observer after two weeks.
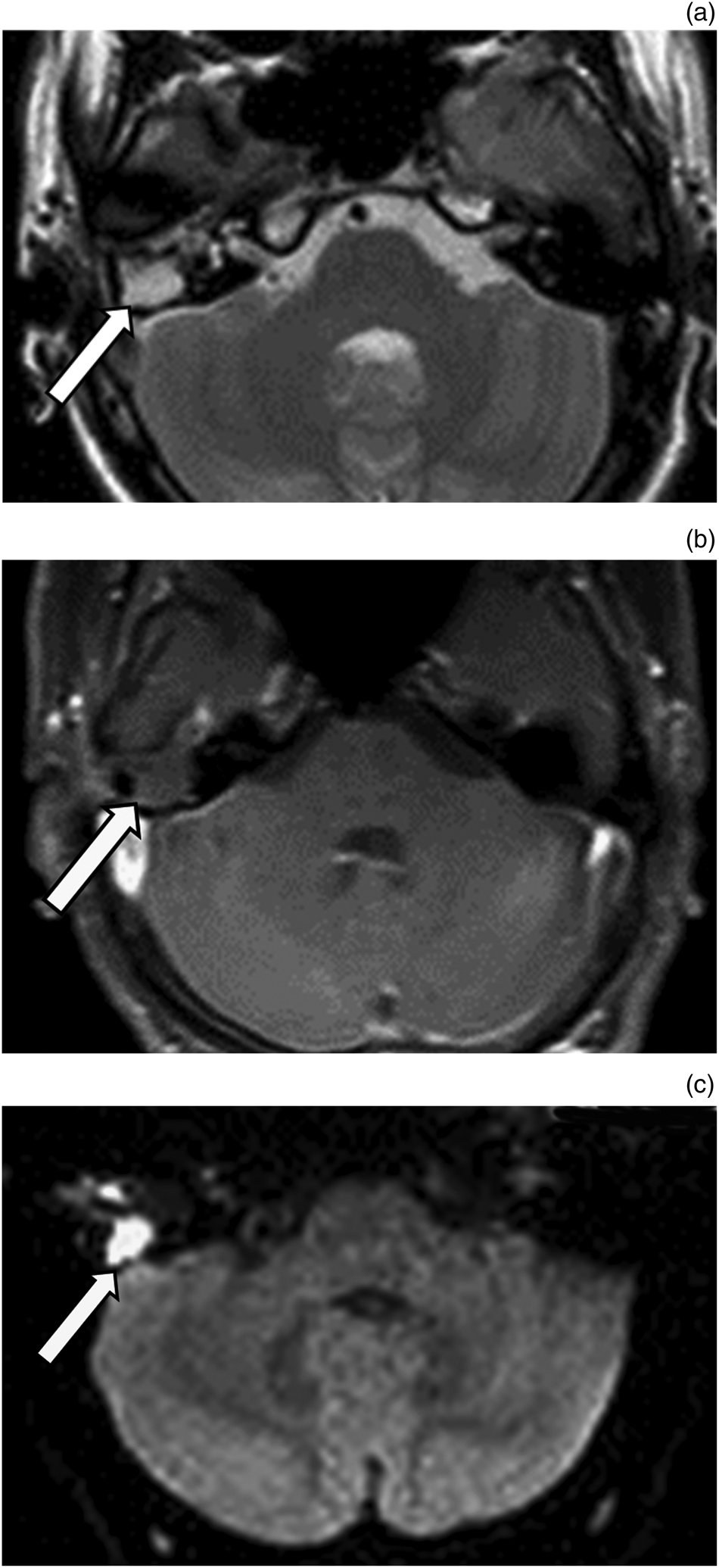
Fig. 1. Recurrent cholesteatoma. (a) Axial, T2-weighted magnetic resonance imaging scan shows hyperintense lesion (arrow) in the right middle-ear cavity. (b) Axial, contrast-enhanced magnetic resonance imaging scan of petrous bone shows no enhancement of the lesion (arrow) in the right middle ear. (c) Axial, diffusion-weighted imaging scan shows unrestricted diffusion of the lesion (arrow) with high signal intensity.
Statistical analysis
All statistical analyses were performed with SPSS software, version 22 (SPSS, Chicago, Illinois, USA). The sensitivity, specificity, accuracy, positive predictive value and negative predictive value for diffusion-weighted imaging and delayed contrast MRI, for both sets of readings, were calculated. The kappa statistic (and 95 per cent confidence interval (CI)) values were calculated to estimate the proportion of agreement between both sets of readings for diffusion-weighted imaging and delayed contrast MRI. The Κ values were interpreted as follows: Κ values between 0.61 and 0.80 represented good agreement, and Κ values between 0.81 and 1.00 represented excellent agreement. A p value of less than 0.05 was considered to indicate a statistically significant difference.
Results
The final diagnosis was recurrent cholesteatoma in 38 patients and granulation tissue in 18 patients. Table 1 shows the detection and reliability of diffusion-weighted imaging and delayed contrast MRI in the differentiation of recurrent cholesteatoma from granulation tissue.
Table 1. Detection and reliability of DWI and delayed contrast MRI in differentiation of recurrent cholesteatoma from granulation tissue

DWI = diffusion-weighted imaging; MRI = magnetic resonance imaging; PPV = positive predictive value; NPV = negative predictive value; CI = confidence interval
On diffusion-weighted imaging, cholesteatoma was associated with unrestricted diffusion in 36 patients and restricted diffusion in 2 patients at both readings. Granulation tissue was associated with unrestricted diffusion in 16 and 17 patients at each reading respectively, and with restricted diffusion in only 1 and 2 patients at each reading respectively. There was good intra-observer agreement for both sets of diffusion-weighted imaging readings in the detection of cholesteatoma (Κ = 0.72, p = 0.001, 95 per cent CI = 0.52–0.91).
The detection of cholesteatoma on diffusion-weighted imaging had a sensitivity of 94.7 and 94.7 per cent, specificity of 94.4 and 88.9 per cent, accuracy of 94.6 and 92.8 per cent, positive predictive value of 97.3 and 94.7 per cent, and negative predictive value of 89.5 and 88.9 per cent, respectively, for the two sets of readings.
The smallest diameter of cholesteatoma on diffusion-weighted imaging was 2.1 mm and the largest was 10.3 mm. The mean cholesteatoma diameter was 7.7 ± 1.8 mm (range, 2.1–10.2 mm) and 7.9 ± 1.8 mm (range, 2.1–10.3 mm) for each set of readings, with excellent intra-observer agreement (Κ = 0.994, p = 0.001).
The detection of cholesteatoma on delayed contrast MRI had a sensitivity of 81.6 and 78.9 per cent, specificity of 77.8 and 66.7 per cent, accuracy of 80.4 and 75.0 per cent, positive predictive value of 88.6 and 83.3 per cent, and negative predictive value of 66.7 and 60.0 per cent, respectively, at each reading. There was fair intra-observer agreement for both sets of delayed contrast MRI readings in the detection of cholesteatoma (Κ = 0.57, p = 0.001, 95 per cent CI = 0.35–0.79).
Discussion
The main finding of this study is that diffusion-weighted imaging is a reliable method for differentiating recurrent cholesteatoma from granulation tissue. There was good intra-observer agreement for diffusion-weighted imaging in the detection of cholesteatoma and excellent intra-observer agreement in measuring the largest diameter of cholesteatoma.
Cholesteatoma was associated with unrestricted diffusion with high signal intensity compared to brain parenchyma in this study. The high signal intensity of cholesteatoma on diffusion-weighted imaging may be attributed to the combination of unrestricted diffusion and ‘T2 shine-through’. Unrestricted diffusion for cholesteatoma is the result of its keratin content, and the T2 shine-through effect occurs in tissues with high T2 signal intensity.Reference Delrue, De Foer, van Dinther, Zarowski, Bernaerts and Vanspauwen20–Reference Nash, Kalan, Lingam and Singh25
In our investigation, diffusion-weighted imaging showed unrestricted diffusion in patients with recurrent cholesteatoma, which may be attributed to the small size of the lesions (less than 2 mm). In addition, granulation tissue revealed unrestricted diffusion mistaken as recurrent cholesteatoma because of the associated acute otitis media. Previous studies have reported the causes of false-negative findings of cholesteatoma as: small cholesteatoma (less than 2–3 mm), auto-atticotomy (epithelial lined sac), cholesteatoma aspiration on microsuction and patient motion artefacts. The causes of false-positive findings of cholesteatoma relate to the presence of: acute otitis media, bone powder, scar tissue, a Silastic sheet, and cholesterol granuloma at the bed of the middle ear that is mistaken for cholesteatoma.Reference Lingam, Nash, Majithia, Kalan and Singh21–Reference Steens, Venderink, Kunst, Meijer and Mylanus26
Diffusion-weighted imaging showed high sensitivity and specificity for the detection of cholesteatoma in the current study. A previous meta-analysis reported that diffusion-weighted imaging had a pooled sensitivity and specificity of 0.91 per cent (95 per cent CI = 0.87–0.95) and 0.92 per cent (95 per cent CI = 0.86–0.96), respectively, for the detection of cholesteatoma.Reference Lingam and Bassett22 Another meta-analysis reported ranges for sensitivity, specificity, positive predictive value and negative predictive value, for diffusion-weighted imaging in the detection of cholesteatoma, of 43–92 per cent, 58–100 per cent, 50–100 per cent and 64–100 per cent, respectively.Reference van Egmond, Stegeman, Grolman and Aarts24
In our investigation, the smallest diameter of cholesteatoma detected with diffusion-weighted imaging was 2.1 mm. It has previously been reported that diffusion-weighted imaging can detect small cholesteatoma up to 2–3 mm in diameter; however, large cholesteatoma up to 5 mm may be missed because of the lack of necessary keratin needed to show restricted diffusion.Reference Bazzi, Wong, Jufas and Patel19–Reference Lingam and Bassett22
The capability of delayed contrast MRI for detecting cholesteatoma was poor in the current study. Previous studies have reported that the diagnostic performance of delayed contrast MRI in the detection of cholesteatoma is extremely variable, with overall sensitivity and specificity values of 14–90 per cent and 55–100 per cent, respectively.Reference Khemani, Singh, Lingam and Kalan5 Granulation tissue may be associated with delayed contrast enhancement, and cholesteatoma may be evident as a non-enhanced lesion. Delayed contrast MRI is a time-consuming examination and can pose a challenge to the service provider within a busy department.Reference Razek, Ghonim and Ashraf6–Reference Abdel Razek and Huang10
• Cholesteatoma detection on diffusion-weighted imaging had sensitivity of 94.7 and 94.7 per cent, specificity of 94.4 and 88.9 per cent, and accuracy of 94.6 and 92.8 per cent
• There was good intra-observer agreement for both sets of diffusion-weighted imaging readings
• Cholesteatoma detection on delayed contrast magnetic resonance imaging (MRI) had sensitivity of 81.6 and 78.9 per cent, specificity of 77.8 and 66.7 per cent, accuracy of 80.4 and 75.0 per cent
• Intra-observer agreement for both sets of delayed contrast MRI readings was fair (Κ = 0.57, p = 0.001)
• The smallest cholesteatoma diameter on diffusion-weighted imaging was 2.1 mm, with excellent intra-observer agreement (Κ = 0.994, p = 0.001)
This study applied non-echo-planar diffusion-weighted imaging for the evaluation of cholesteatoma, in order to obtain high-resolution scans with thin slices (2 mm), and hence detect small cholesteatoma. Previous studies have reported that non-echo-planar diffusion-weighted imaging is more reliable than echo-planar diffusion-weighted imaging, with high spatial resolution, and no air–bone susceptibility artefacts and distortion at the temporal bones.Reference Muzaffar, Metcalfe, Colley and Coulson23–Reference Razek and Castillo28 One study reported that the echo-planar diffusion-weighted imaging had sensitivity of 71.8 per cent, specificity of 89.3 per cent, positive predictive value of 93.3 per cent and negative predictive value of 73.3 per cent, and that non-echo-planar diffusion-weighted imaging had sensitivity 89.7 per cent, specificity of 94.5 per cent, positive predictive value of 96.5 per cent and negative predictive value of 80.4 per cent, for the detection of cholesteatoma, with significantly improved sensitivity for non-echo-planar diffusion-weighted imaging (p = 0.02).Reference Muzaffar, Metcalfe, Colley and Coulson23
There are a few limitations to this study. First, it included only a small number of patients; multicentre studies are required, with validation based upon a large number of patients. Second, this study involved diffusion-weighted imaging using a 1.5 Tesla scanner. Further studies using diffusion tensor imaging,Reference Abdel Razek29–Reference Razek, El-Serougy, Abdelsalam, Gaballa and Talaat32 dynamic contrast MRIReference Abdel Razek, Samir and Ashmalla33 on a higher Tesla scanner,Reference Abdel Razek, Elkhamary, Al-Mesfer and Alkatan34 and combined with CT,Reference Foti, Beltramello, Minerva, Catania, Guerriero and Albanese35 will improve the results.
Conclusion
Diffusion-weighted imaging is a reliable method for differentiating recurrent cholesteatoma from granulation tissue after intact canal wall mastoidectomy.
Competing interests
None declared




