Introduction
Open partial laryngectomy is an intervention provided to selected patients who present with T1–T3 primary, residual or recurrent laryngeal cancer.Reference Paleri, Thomas, Basavaiah, Drinnan, Mehanna and Jones1, Reference Thomas, Drinnan, Natesh, Mehanna, Jones and Paleri2 The most commonly used open partial laryngectomy option used for glottic and supraglottic tumours is a supracricoid partial laryngectomy, which can take one of two forms: cricohyoidopexy or cricohyoidoepiglottopexy.Reference Bron, Brossard, Monnier and Pasche3, Reference Laccourreye, Laccourreye, Menard, Weinstein and Brasnu4 The other option is a vertical partial laryngectomy, used primarily for unilateral glottic tumours; although traditionally no formal reconstruction is employed, temporoparietal free flap, cartilage graft and buccal mucosal graft have been employed following vertical partial laryngectomy, with the aim of a better voice outcome.Reference Gilbert, Goldstein, Guillemaud, Patel, Higgins and Enepekides5 All of these surgical approaches and reconstruction methods alter the capacity of the larynx to achieve effective phonation and airway protection during swallow.Reference Lewin, Hutcheson, Barringer, May, Roberts and Holsinger6
Beyond the site and size of the tumour, many other components impact on functional voice and swallow outcomes. There is limited literature exploring selection criteria and therapeutic interventions to optimise outcomes. Additionally, rehabilitation practices show significant international and national variability created by the availability, or lack thereof, of rehabilitation experts, facilities and care pathways.
In keeping with the findings from two recent systematic reviews,Reference Lips, Speyer, Zumach, Kross and Kremer7, Reference Schindler, Pizzorni, Mozzanica, Fantini, Ginocchio and Bertolin8 this paper was created to support the development of guidelines for managing post-operative rehabilitation and standardising care for this specific cohort of patients.
Selection criteria and functional assessment
Given the dwindling numbers of patients who undergo open partial laryngectomy, robust pre-operative selection criteria are vitally important to ensure good outcomes. Lung function in particular should be assessed pre-operatively, as significant aspiration is a predictable component of post-operative rehabilitation, and has been shown to have potentially long-term adverse effects.Reference Bagwell, Leder and Sasaki9 This assessment should include a detailed case history about any chronic lung disease, and/or recent infections or hospital admissions as a result of compromised lung function.
Patients who require the use of home oxygen or continuous positive airway pressure overnight (other than for snoring) are also very unlikely candidates for supracricoid partial laryngectomy or vertical partial laryngectomy, owing to the likely irreversible risk of lung damage following persistent aspiration. In addition, the literature has revealed an association between patients with chronic obstructive pulmonary disease undergoing open partial laryngectomy and an increased risk of in-hospital mortality, compared to those undergoing total laryngectomy.Reference Sylvester, Marchiano, Park, Baredes and Eloy10
Currently, there are no standardised measures that can reliably predict which patients will go on to develop pulmonary complications following this surgery. Indicators such as the ability to climb stairs have been explored previously in literature; however, these have not been sensitive enough to predict outcomes.Reference Chow, Block and Friedman11
Research has begun to explore the negative impact of open partial laryngectomy on maximum phonation time, and the potential severity of restrictive and obstructive respiratory function post-surgery.Reference Demir12 Whilst these are exploratory data, they reiterate the requirement for holistic assessment and consideration of lung function pre-operatively.
The general health of the patient should not be overlooked in the selection phase. General frailty, diabetes, movement disorders and any cognitive issues need to be balanced against the rigorous rehabilitation regimes that will be expected of the patient in the post-operative period. Any co-morbidity that may impact on the patient's ability to mobilise after surgery, heal effectively, or engage in robust physiotherapy and swallow therapy should be identified and optimised if possible. The patient should be made aware that their swallow rehabilitation will begin in the early post-operative phase; its success is interrelated with tracheostomy decannulation, return to oral intake and voice function. Patients’ understanding and engagement in this process is paramount.
The capacity and capability of the patient to tolerate post-operative rehabilitation needs to be assessed by the multidisciplinary team (MDT) on an individual basis. Collaborative discussion between the surgeon, speech and language therapist, and the patient needs to take place from the outset, so that the patient is aware of what is expected of them in the post-operative phase. This also allows the clinical team to make an informed decision regarding their likely tolerance and the pace of post-operative rehabilitation.
Baseline measures of function and quality of life are useful to collect at this point, to quantify change through subsequent rehabilitation, and to identify areas of improvement the patient or clinician feels would benefit from targeted intervention. These may include voice quality, swallow competence or exploring the psychosocial facets of change following partial laryngeal surgery.
No specific outcome measures have been standardised for this patient group; however, commonly used measures include: the MD Anderson Dysphagia Inventory,Reference Chen, Frankowski, Bishop-Leone, Hebert, Leyk and Lewin13 the 100 ml water swallow test,Reference Patterson, Hildreth, McColl, Carding, Hamilton and Wilson14 the Normalcy of Diet scale,Reference List, Ritter-Sterr and Lansky15 the University of Washington Quality of Life Scale,Reference Rogers, Gwanne, Lowe, Humphris, Yueh and Weymuller16 the European Organization for Research and Treatment of Cancer 30-item quality of life questionnaire (‘QLQ-C30’),Reference Aaronson, Ahmedzai, Bergman, Bullinger, Cull and Duez17 the Voice Handicap Index,Reference Jacobson, Johnson, Grywalski, Silbergleit, Jacobson and Benninger18 and the grade, roughness, breathiness, asthenia and strain (‘GRBAS’) scale.Reference Hirano19
Rehabilitation principles
It is of fundamental importance that the speech and language therapist providing swallow and voice rehabilitation for this patient group is appropriately skilled and resourced. The therapeutic intervention required is complex and iterative in nature. It relies on advanced skills developed through an understanding of the evidence base, clinical experience, and collaborative working with the surgical team and wider MDT.
Rehabilitation usually begins in the pre-operative phase, with information on postural adaptations and exercise regimens that are likely to be recommended by the speech and language therapist after surgery. We suggest that exercises are carried out frequently until the planned surgery, with the aim of supporting the patient to become adept at the exercise. It is not expected, and it is unlikely, that the patient will increase or improve muscle tone by the time they undergo surgery; however, there are other benefits to these interventions.
The super-supraglottic swallow is frequently used to orientate the patient to the concept and feeling of deliberate vocal fold adduction, and to help create a sphincteric supraglottic function.Reference Logemann, Pauloski, Rademaker and Colangelo20 This deliberate tightening of the area above the larynx seems to help prevent and manage intra-swallow aspiration, and enables the patient to exert some control over the co-ordination of their swallow, increasing the duration of the airway closure.Reference Logemann, Gibbons, Rademaker, Pauloski, Kahrilas and Bacon21 Teaching this skill can be useful prior to surgery, as the patient becomes aware of the rationale of the rehabilitation. This also helps create the sensation of how voice will be created in the case of the supracricoid partial laryngectomy, where the arytenoids approximate at the suprahyoid epiglottis, facilitating voice generation via vibration of mucosa between the surfaces (Figure 1). In vertical partial laryngectomy, the approximation of the true fold against the reconstructed or reassembled new glottis of the larynx results in the mode of phonation where the arytenoid on the unaffected side is recruited and facilitates glottic closure (Figure 2).
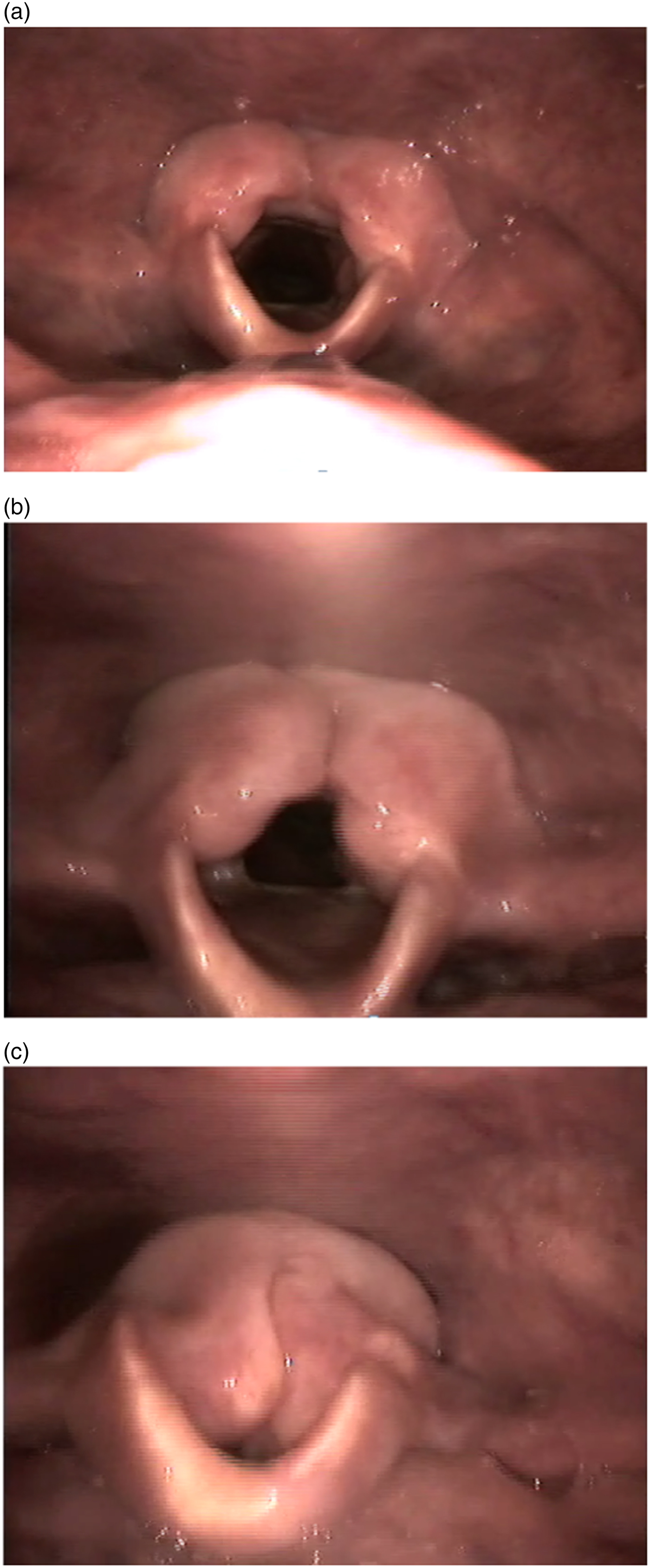
Fig. 1. Sequential frame grabs of videolaryngoscopic examination during phonation after supracricoid partial laryngectomy, showing: (a) the resting position; (b) the onset of phonatory effort; and (c) the arytenoids approximate to recreate the vibrating surface. The neo-glottis comprises two mobile arytenoids.

Fig. 2. Sequential frame grabs of videolaryngoscopic examination during phonation after vertical partial laryngectomy, showing: (a) the resting position; (b) the onset of phonatory effort; and (c) glottis closure achieved by recruitment of one functioning arytenoid. Note the relative immobility of the right hemilarynx. The neo-glottis comprises the unaffected true vocal fold and the reconstructed contralateral glottis.
Providing the patient with visual feedback using endoscopy is also of value, as vocal fold closure is problematic to assess with clinical assessment alone, and it can enhance patient learning.Reference Denk and Kaider22 The patient and team should be prepared to tolerate the inevitable phase of frank aspiration that accompanies swallow rehabilitation, which is an expected component of the rehabilitation trajectory.
Acute dysphagia rehabilitation and compensatory strategies may include but are not limited to: deliberately closing the laryngeal inlet pre-swallow and cough releasing after swallow (super-supraglottic swallow);Reference Logemann, Pauloski, Rademaker and Colangelo20 improving tongue base to posterior pharyngeal wall retraction and tension, to enhance bolus drive (Masako manoeuvre or effortful swallow);Reference Fujiu and Logemann23 and improving hyolaryngeal excursion and anterior tilt (Mendelsohn manoeuvre or Shaker exercise).Reference Logemann, Rademaker, Pauloski, Kelly, Stangl-McBreen and Antinoja24, Reference Lazarus, Logemann and Gibbons25
Voice rehabilitation and rehabilitation strategies may include: breath hold and release, to develop sphincteric action, subglottic pressure or valving; inflating air in the cheeks, with breath hold and release (Valsalva manoeuvre); accented or voiced fricatives, to encourage phonation; semi-occluded techniques; diaphragmatic breath support; and vocal hygiene.
In the first 1–2 post-operative days, the patient should be told to spit out their saliva and avoid phonation. The cuff on the tracheostomy should remain inflated for the first 2 days, to limit the volume and frequency of aspiration, and to reduce the potential trauma caused by coughing and constant expectoration of secretions. Discussion with the speech and language therapist and surgeon influences the point at which the cuff will be deflated, and the patient will be encouraged and shown how to swallow most effectively. As soon as this process can happen without compromising the surgical site, therapy needs to begin, to reduce the risk of disuse atrophy and encourage compensatory strategy. Instrumental evaluation, using either videofluoroscopy or fibre-optic endoscopic evaluation of swallow, is of value and can be used to provide biofeedback.
Table 1 shows the range of time taken for the various rehabilitative milestones at a single centre in the UK, for 11 patients. The functional outcome data were gathered from the Queen Elizabeth Hospital Birmingham.
Table 1. Time taken for the various rehabilitative milestones at a single UK centre

The data represent the range of time taken for each rehabilitative milestone, in days. The functional outcome data were gathered from the Queen Elizabeth Hospital Birmingham. *n = 7; †n = 4
Training needs and centralisation of services
The successful provision of a supracricoid partial laryngectomy and vertical partial laryngectomy service relies on more than the operative skill of the surgical team, or the rehabilitative capabilities and capacity of the speech and language therapists. The nature of this procedure requires expertise grounded in experience and exposure to this intervention, along with a robust ability to reflect clinically and adapt practice, facilitated by the centralisation of clinical services. This approach results in fewer clinical teams providing higher volumes of this type of surgery to patients, so that clinicians can achieve the best possible outcomes.
This concept raises valuable questions about increasing travelling distance for patients and relatives,Reference Stitzenberg, Sigurdson, Egleston, Starkey and Meropol26 and the deskilling of clinical teams who may not provide this surgery. However, the fundamental importance of optimising disease-free survival and improving clinical outcomes cannot be overlooked. Research has demonstrated that centralisation of oesophageal cancer surgery can reduce length of stay and post-operative morbidity, and significantly improve survival.Reference Wouters, Karim-Kos, le Cessie, Wijnhoven, Stassen and Steup27 Clinical trends towards better outcomes have been demonstrated in head and neck cancer too.Reference Nouraei, Middleton, Hudovsky, Darzi, Stewart and Kaddour28
It is also useful to consider the soft components of interdisciplinary working and the infrastructure that supports this type of surgical procedure. The MDT meetings should be a forum for careful discussion and deliberation around the indications for this procedure. Whilst time and the volume of cases may impact on the practicalities of engaging in this type of discussion, time should be ring-fenced to ensure comprehensive evaluation and treatment plan creation. Similarly, the team should engage in open and reflective discussion about ways they may develop and improve services. The surgical and rehabilitative team need to work closely together to ensure that best practice is achieved and that regular training opportunities to the MDT are provided.
Suggested rehabilitation care pathway
Figure 3 shows a model care pathway that provides practical suggestions regarding rehabilitation and optimising functional outcomes. It is based on research evidence where available, and the experience of the authors.

Fig. 3. Suggested care pathway for rehabilitation after open partial laryngectomy. MDADI = MD Anderson Dysphagia Inventory; SLT = speech and language therapist; MDT = multidisciplinary team; NG = nasogastric
Future developments
This paper has identified key issues and recommendations for the provision of open partial laryngectomy services. A key finding was the paucity of evidence regarding optimal rehabilitation pathways. It is unlikely that this situation will change given the challenge of attempting to conduct randomised controlled trials with this discreet and heterogeneous group of patients. Instead, it may be more pragmatic to consider collecting data from the UK centres that carry out this intervention, to compare outcomes and rehabilitation pathways, and ultimately to develop standardised programmes. The authors also recommend limiting the number of centres within the UK that carry out this surgical approach, in order to maintain and develop the confidence and competence of the surgical and rehabilitative teams managing this group of patients.
Competing interests
None declared






