Introduction
The incidence of human papillomavirus (HPV) associated oropharyngeal cancer continues to increase,Reference Chaturvedi, Engels, Pfeiffer, Hernandez, Xiao and Kim1 and HPV status has been found to be a strong prognostic factor for survival.Reference Ang, Harris, Wheeler, Weber, Rosenthal and Nguyen-Tan2–Reference Lassen, Eriksen, Hamilton-Dutoit, Tramm, Alsner and Overgaard4 The Radiation Therapy Oncology Group ‘RTOG 0129’ trial evaluated a large cohort of patients in a randomised controlled trial, in which patients received concurrent chemotherapy and radiotherapy (RT). The study found that patients with HPV-positive tumours had a three-year overall survival rate of 82 per cent, compared with 57 per cent in patients with HPV-negative tumours.Reference Ang, Harris, Wheeler, Weber, Rosenthal and Nguyen-Tan2
The standard combination of chemotherapy and RT (70 Gy), established by the Head and Neck IntergroupReference Adelstein, Li, Adams, Wagner, Kish and Ensley5 and French Head and Neck Oncology and Radiotherapy Group (‘GORTEC’)Reference Denis, Garaud, Bardet, Alfonsi, Sire and Germain6 for stage III or IV oropharyngeal cancer, can result in grade three to four toxicity rates of 56–89 per cent, with toxicities including mucositis, dysphagia and leukopenia. Long-term side effects include grade three to four pharyngeal and/or laryngeal toxicity, and a requirement for a feeding tube.Reference Machtay, Moughan, Trotti, Garden, Weber and Cooper7
Given the overall excellent prognosis of HPV-positive tumours, there is significant interest in de-intensifying treatment, with the goal of reducing both acute and chronic toxicity in this population of younger and healthier patients.
A recent phase II study showed an excellent pathological complete response rate of 86 per cent after treatment with a reduced radiation dose of 60 Gy, weekly low-dose cisplatin and planned neck dissection.Reference Chera, Amdur, Tepper, Qaqish, Green and Aumer8 Another phase II trial, by the Eastern Cooperative Oncology Group (ECOG 1308), evaluated dose de-escalation with 54 Gy RT in patients with a complete clinical response to induction chemotherapy. The results showed excellent rates of progression-free survival and overall survival of 80 per cent and 94 per cent, respectively.Reference Marur, Li, Cmelak, Gillison, Zhao and Ferris9 In particular, patients with favourable features (lower than tumour (T) stage T4, lower than nodal (N) stage N2c, and 10 pack-year or fewer smoking history) treated with a radiation dose of 54 Gy or lower had two-year overall survival and progression-free survival rates of 96 per cent and 96 per cent, respectively. A third phase II study also evaluated dose de-escalation with 54 Gy radiation in patients with a complete or partial response to induction chemotherapy and 60 Gy in patients with less than partial or no responses.Reference Chen, Felix, Wang, Hsu, Basehart and Garst10 That study reported an excellent two-year progression-free survival rate of 92 per cent. Several additional clinical trials evaluating treatment de-intensification with lower radiation doses are ongoing.
In this hospital-based population study, we evaluated survival outcomes in relation to RT dose in patients with HPV-positive tumours.
Materials and methods
The National Cancer Database is a joint programme of the American College of Surgeons Commission on Cancer and the American Cancer Society. The National Cancer Database is a nationwide, facility-based, comprehensive clinical surveillance resource oncology dataset that captures 70 per cent of all newly diagnosed malignancies in the USA annually.Reference Winchester, Stewart, Phillips and Ward11 Access to the de-identified National Cancer Database file was granted to the listed authors. Institutional review board approval was obtained.
We evaluated patients with non-metastatic, T1–3, N0–2c oropharyngeal carcinoma, who received definitive RT with known radiation doses shown in the National Cancer Database (2010–2014) (Figure 1). Patients with no vital status or less than six months of follow up were excluded. Patients were also excluded if they had had prior cancer, non-squamous histology findings or undergone definitive surgery, or if chemotherapy use, education and income were unknown. Definitive surgery was determined using surgical codes from the Facility Oncology Registry Standards manual.
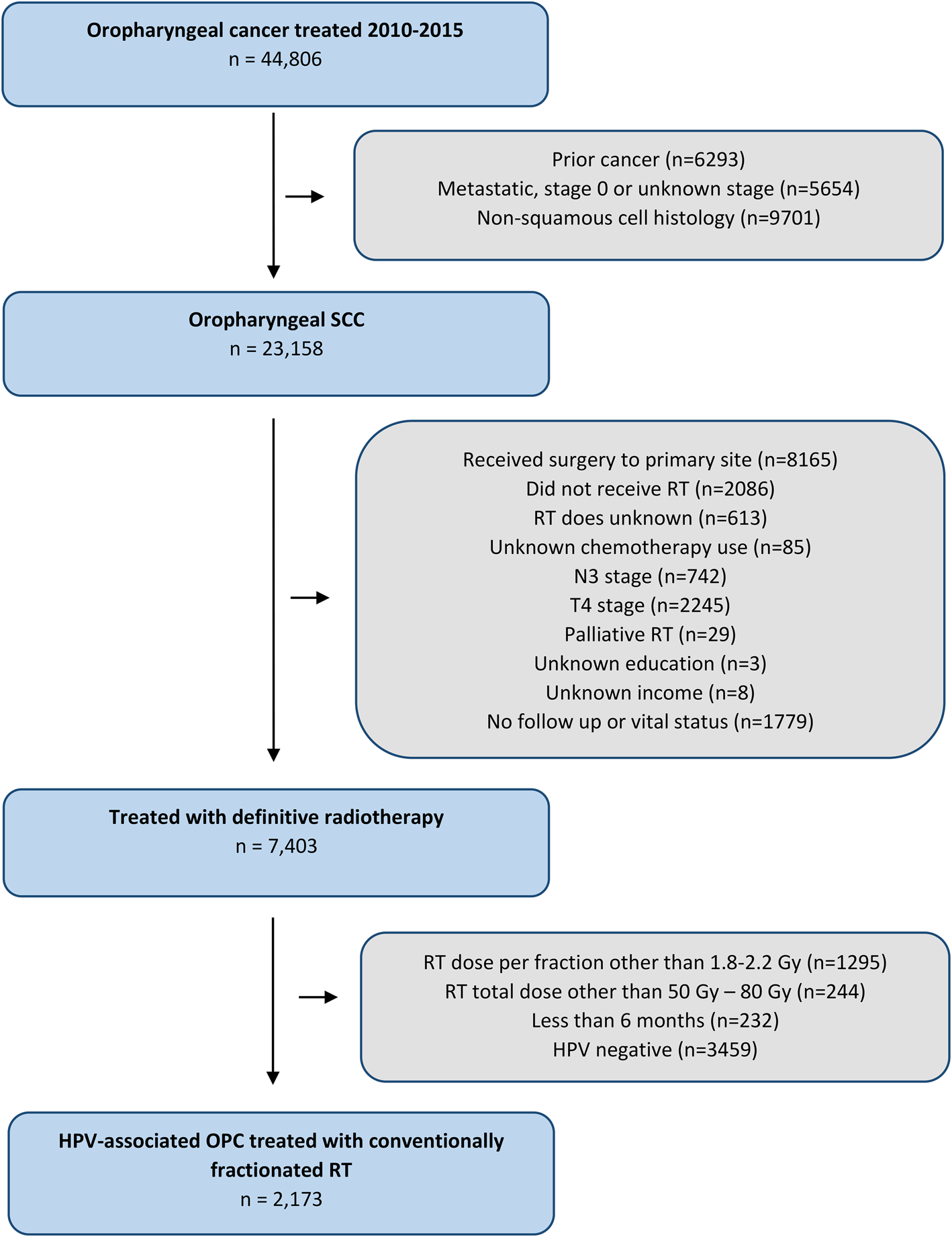
Fig. 1. Consolidated Standards of Reporting Trials (‘CONSORT’) diagram detailing the study inclusion criteria. SCC = squamous cell carcinoma; RT = radiotherapy; N = node; T = tumour; HPV = human papillomavirus; OPC = oropharyngeal carcinoma
Total radiation doses were calculated as the sum of the recorded regional and boost radiation doses. Dose per fraction was determined from the total radiation dose and the recorded total number of fractions. Patients were stratified by radiation dose (i.e. those receiving 66 Gy or more, and those receiving 50 Gy or more but less than 66 Gy). The threshold of 66 Gy was used because doses of 66 Gy in 2.2 Gy per fractionReference Eisbruch, Harris, Garden, Chao, Straube and Harari12 and 70 Gy in 2.0 Gy per fraction are considered to be the standard of care treatment dose for oropharyngeal cancer according to the National Comprehensive Cancer Network (version 3.2019) and consensus guidelines.Reference Quon, Vapiwala, Forastiere, Kennedy, Adelstein and Boykin13,Reference Sher, Adelstein, Bajaj, Brizel, Cohen and Halthore14 An upper threshold of 80 Gy was used. Patients who received radiation as a palliative measure were excluded from analysis. Patients were included if they received a radiation dose per fraction of 180 cGy to less than 220 cGy.
Statistical analysis of categorical data was performed with the chi-square test. Survival curves were plotted using the Kaplan–Meier method. The effect of RT dose was evaluated using Cox proportional hazards modelling, adjusted for clinicopathological, demographic and socioeconomic factors.
Socioeconomic and demographic factors included age, race, insurance status, facility type, distance from treatment centre, median income and education. Education was measured according to the number of adults in the patient's zip code who did not graduate from high school. Clinicopathological factors included tumour (T) category, nodal (N) category, HPV subtype, Charlson/Deyo co-morbidity score, chemotherapy and year of diagnosis. Smoking status, and details regarding chemotherapy dosing or type, are not recorded in the National Cancer Database. Staging was based on the American Joint Committee on Cancer, seventh edition.
Survival analysis was performed using the adjusted staging of the International Collaboration on Oropharyngeal Cancer Network for Staging (‘ICON-S’), given their improved use as a prognosticator in HPV-related oropharyngeal cancer.Reference O'Sullivan, Huang, Su, Garden, Sturgis and Dahlstrom15 Thus, in our analysis, ipsilateral lymph node involvement with categories N1, N2a and N2b were grouped together (International Collaboration on Oropharyngeal Cancer Network for Staging N1).
The primary outcome was overall survival. Details regarding patterns of disease recurrence (local-regional or distant relapse) were not available. Bivariate analysis using the chi-square test was used to analyse categorical variables with respect to differing RT dose levels. Binomial logistic regression was performed to identify factors that predicted the receipt of RT with lower doses (50 Gy to less than 66 Gy). Survival curves were plotted using the Kaplan–Meier method, and the log-rank test was used to determine statistical significance. The Cox proportional hazards model was used to identify factors associated with overall survival in multivariate analysis models. All tests were two-sided, and a p-value of less than 0.05 was regarded as statistically significant. All statistical analyses were performed using R statistical packages.
Results
A total of 2173 patients with HPV-positive oropharyngeal carcinoma were included for analysis. Patient and disease baseline characteristics are summarised in Table 1. The median age was 57 years (range, 22 to 90 years). Ninety-four per cent of patients had stage III–IV disease, 87 per cent had a Charlson/Deyo score of 0, and 90 per cent received chemotherapy. The HPV positivity was documented as high risk type 16 in 65 per cent of patients, as type not stated in 25 per cent and as another subtype in 10 per cent. Among these patients, 124 (6 per cent) received an RT dose of 50 Gy to less than 66 Gy. All patients received radiation doses of 50 Gy to less than 80 Gy. Additionally, all patients received radiation fraction sizes of 180 cGy to less than 220 cGy. On multiple logistic regression, no factors were associated with an increased likelihood of receiving radiation doses of 50 Gy to less than 66 Gy (Table 2).
Table 1. Baseline patient and disease characteristics

*n = 2049; †n = 124. ‡Indicates statistical significance (p < 0.05). **Number of adults in patient's zip code who did not graduate from high school. RT = radiotherapy; AJCC = American Joint Committee on Cancer; NA = not applicable; HPV = human papillomavirus
Table 2. Multiple logistic regression for RT dose of 50 to less than 66 Gy

RT = radiotherapy; OR = odds ratio; CI = confidence interval
With a median follow up of 33.8 months (range, 6.0–83.0 months), the entire cohort of patients with HPV-positive tumours had a 3-year overall survival rate of 88.6 per cent (95 per cent confidence interval (CI) = 87.1–90.1 per cent). A total of 254 deaths were recorded during the study period (12 per cent). Patients receiving a radiation dose of 66 Gy or more or 50 Gy to less than 66 Gy had a three-year overall survival rate of 88.5 per cent (95 per cent CI = 87.0–90.1 per cent) and 89.9 per cent (95 per cent CI = 84.0–96.2 per cent), respectively (log-rank p = 0.57) (Figure 2).

Fig. 2. Kaplan–Meier survival estimates for patients who received total radiation doses of 66 Gy or more, or 50 to less than 66 Gy (log-rank p = 0.57).
On univariate survival analysis, older age, advanced T category, government-type insurance and not receiving chemotherapy increased the risk of all-cause death (Table 3). A radiation dose of 50 Gy to less than 66 Gy, race, N category, a Charlson/Deyo score, and number of nodes examined were not significant predictors for survival.
Table 3. Prognostic factors for overall survival

Multivariate model includes age, tumour (T) category, nodal (N) category and parameters found to be at 10 per cent significance level in the univariate procedure. *Indicates statistical significance (p < 0.05). HR = hazard ratio; CI = confidence interval
Multivariate Cox analysis included age, T category, N category and additional significant parameters at the 10 per cent significance level from the univariate analysis. On multivariate analysis, radiation doses of 50 Gy to less than 66 Gy (hazard ratio = 0.95, 95 per cent CI = 0.52–1.74, p = 0.86) did not independently predict increased mortality risk when compared with doses of 66 Gy or more (Table 3). Multivariate analysis also revealed that advanced T stage (T3) and not having received chemotherapy were independent predictors of increased mortality.
Discussion
In this hospital-based analysis of 2173 patients with T1–3 and N0–2c HPV-positive oropharyngeal cancer, we found that definitive RT doses of 50 Gy to less than 66 Gy did not have a statistically significant impact on survival when compared with doses of 66 Gy or more, on multivariate analysis. The overall survival rate at three years in patients receiving 50 Gy to less than 66 Gy was excellent, at 90 per cent. These survival outcomes are similar to those of recent phase II studies in which patients received treatment de-escalation with RT doses of 54 to 60 Gy.Reference Marur, Li, Cmelak, Gillison, Zhao and Ferris9,Reference Chen, Felix, Wang, Hsu, Basehart and Garst10
These results are consistent with the literature in which patients with HPV-positive oropharyngeal cancer have been found to exhibit an enhanced response to radiation with a more dramatic rapid initial regression than those with HPV-negative tumours.Reference Chen, Li, Beckett, Zhara, Farwell and Lau16 The exact mechanism of the HPV-mediated treatment response is unclear. The HPV infection results in viral products E6 and E7, which leads to the suppression of p53 and retinoblastoma protein (pRb). However, in vitro and in vivo studies have shown that high-level expression of E6 oncogene results in radiation resistance,Reference Hampson, El Hady, Moore, Kitchener and Hampson17 while reducing the expression of E6 and E7 results in increased sensitivity to cisplatin and radiation.Reference Zheng, Zhang and Rao18 Perhaps, the rapid response to treatment is related to the viral antigens expressed, which allow for an enhanced host immune response.Reference Spanos, Nowicki, Lee, Hoover, Hostager and Gupta19,Reference Williams, Lee, Elzey, Anderson, Hostager and Lee20
Currently, there are several approaches under investigation that focus on de-intensifying treatment with decreases in radiation dose.Reference Tam and Hu21 The NRG (acronym derived from the first letters of the following parental groups: National Surgical Adjuvant Breast and Bowel Project, Radiation Therapy Oncology Group, and Gynecologic Oncology Group) Head and Neck Oncology Group is performing a randomised phase II trial (ClinicalTrials.gov identifier: NCT02254278) evaluating chemoradiation (60 Gy in six weeks with concurrent 40 mg/m2 weekly cisplatin for six weeks) compared with accelerated RT alone (60 Gy in five weeks using six fractions per week). The Lineberger Comprehensive Cancer Center is also performing a phase II study (NCT02281955) to evaluate chemoradiotherapy with 60 Gy intensity-modulated RT. Additionally, the Quarterback Trial (NCT01706939) is a randomised, phase III study evaluating chemoradiation with 56 Gy or 70 Gy after induction chemotherapy. Furthermore, our institution is evaluating chemoradiation treatment de-escalation with 60 Gy in patients who experience early tumour shrinkage mid-treatment (NCT03215719). Patients receive an interval scan at four weeks to assess for a good response, defined as more than 40 per cent nodal shrinkage, and patients are stratified according to the treatment received: standard treatment with 70 Gy or a dose-deescalated treatment regimen.
We were unable to evaluate patients who received radiation doses of less than 50 Gy as patients would not typically be treated definitively with those doses. A pilot study is currently evaluating a radiation dose of 30 Gy given with concurrent chemotherapy in select patients with HPV-positive oropharyngeal carcinoma (NCT00606294). Patients qualify for a dose reduction if they do not demonstrate persistent hypoxia on evaluation with 18F-fluoromisonidazole positron emission tomography imaging. Preliminary results are promising, with a pathological complete response seen in 18 of 19 patients.Reference Riaz, Sherman, Katabi, Leeman, Higginson and Boyle22
The objective of radiation dose reduction in these clinical trials is to allow for clinically significant decreases in radiation doses to normal tissue structures. For instance, use of intensity-modulated RT to spare swallowing structures has been shown to provide potential benefits in patient-reported, observer-rated and objective measures of swallowing.Reference Feng, Kim, Lyden, Haxer, Worden and Feng23 Thus far, limited data are available on quality-of-life outcomes in patients receiving dose de-escalation. The Eastern Cooperative Oncology Group ECOG 1308 study reported excellent quality-of-life outcomes in patients who received dose de-escalation, but it is unclear whether this was due to better tumour selection or treatment effect.Reference Marur, Li, Cmelak, Gillison, Zhao and Ferris9 Therefore, we anticipate the results of the ongoing trials such as the NRG randomised phase II study (ClinicalTrials.gov identifier: NCT02254278), which will be evaluating quality-of-life outcomes using several validated head and neck cancer specific questionnaires.
Our results must be interpreted within the limitations of this study. The exact threshold for RT dose cannot be elucidated from this analysis given the limited number of patients receiving an RT dose of less than 66 Gy. With more patients in a future database analysis, a receiver operating characteristic curve may be more useful in potentially providing a more meaningful threshold. Furthermore, smoking status, chemotherapy details, and cancer-specific outcomes including local-regional control and distant metastasis were not recorded in the National Cancer Database. Cancer-specific outcomes may be particularly important in the HPV-associated patients given the improved prognosis when compared with HPV-negative patients.Reference Fakhry, Zhang, Nguyen-Tan, Rosenthal, El-Naggar and Garden24 Therefore, three-year survival outcomes may not be sufficient in HPV-associated oropharyngeal cancers. Additional limitations of this data analysis include coding errors, incomplete data and selection bias. Regarding HPV status, limitations include non-uniform HPV testing methods, variable proportions of HPV evaluation across institutions, and potential biases in each institution with known versus unknown HPV status. The strength of this study lies in the large cohort of patients evaluated and the radiation details that were evaluated, which included regional radiation dose, boost dose and number of fractions.
• Several ongoing, large randomised trials highlight current interest in treatment de-escalation in human papillomavirus (HPV)-associated oropharyngeal cancer
• A hospital-based analysis was conducted of over 2000 patients with HPV-associated oropharyngeal cancer receiving de-escalated definitive radiotherapy (RT)
• Patients treated with 50 to less than 66 Gy did not have compromised survival when compared to those who received standard RT doses of 66 Gy or more
• These promising results further support patients enrolling into clinical trials for radiation dose de-escalation
• Such dose de-escalation can reduce toxicity and improve long-term quality of life in this patient population who are younger and healthier
Of note, the recent NRG Oncology/RTOG 1016 trial was a negative phase III study that showed inferior survival using treatment de-escalation using cetuximab instead of cisplatin.Reference Gillison, Trotti, Harris, Eisbruch, Harari and Adelstein25 Therefore, the standard of care remains to treat patients with local regionally advanced oropharyngeal carcinoma with standard dose chemoradiotherapy until we have phase III data showing efficacy of RT dose de-escalation.Reference Quon, Vapiwala, Forastiere, Kennedy, Adelstein and Boykin13
In conclusion, in our analysis of over 2173 patients with HPV-positive oropharyngeal carcinoma, RT doses of 50 Gy to less than 66 Gy did not negatively impact survival in these hypothesis-generating data. These results further support patients enrolling into clinical trials for RT dose de-escalation.
Acknowledgement
The National Cancer Database is a joint project of the Commission on Cancer. The data used in the study are derived from a de-identified National Cancer Database file. The Commission on Cancer have not verified and are not responsible for the analytical or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Competing interests
None declared







