Introduction
Ototoxicity is a general term that describes cochlear and vestibular organ damage resulting from exposure to various therapeutic agents or chemical substances.Reference Roland and Rutka 1 Specifically, ototoxicity is the functional impairment and cellular degeneration of the inner-ear tissues caused by therapeutic agents. The most common ototoxic drugs in clinical use include: aminoglycosides, antibiotics, platinum-based chemotherapeutic agents (cisplatin and carboplatin), loop diuretics, macrolide antibiotics and antimalarials.Reference Aslan, Orzan and Santorelli 2
Cisplatin is an effective therapeutic agent for various human cancers, including carcinoma of the head and neck.Reference Kalcioglu, Kizilay, Gulec, Karatas, Iraz and Akyol 3 Ototoxicity and nephrotoxicity are the most common side effects of cisplatin. Intravenous hydration and diuresis effectively decrease the severity of nephrotoxicity. However, there is no defined therapeutic agent to prevent ototoxicity in humans, although some agents have been investigated in animal experiments.Reference Choe, Chinosornvatana and Chang 4 – Reference Kalkanis, Whitworth and Rybak 6
Symptoms related to cisplatin ototoxicity, such as temporary or permanent tinnitus, and progressive and usually irreversible high-frequency hearing loss, have been reported in 4–74 per cent of cases.Reference Hyppolito, de Oliveire, Lessa and Rossato 5 , Reference Biro, Noszek, Prekopp, Nagyiványi, Géczi and Gaudi 7 , Reference Strauss, Towfighi, Lord, Lipton, Harvey and Brown 8 Clinically, this may present as a moderate degree of high frequency sensorineural hearing loss, accompanied by tinnitus. Unilateral hearing loss and temporary threshold shifts are rare, but may be encountered.
Cisplatin-induced ototoxicity is thought to result from increased amounts of toxic free radicals or cell membrane changes leading to decreased intracellular calcium content.Reference Liang, Schulte, Qu, Hu and Shen 9 Animal experiments have revealed that cisplatin ototoxicity primarily targets the outer hair cells, particularly those located in the basal and middle turns of the cochlea.Reference Rybak, Whitworth, Mukherjea and Ramkumar 10 – Reference Devarajan, Savoca, Casteneda, Park, Esteban-Cruciani and Kalinec 12 These studies have also shown damage to Reissner's membrane and the stria vascularis.Reference Rybak, Whitworth, Mukherjea and Ramkumar 10 – Reference Devarajan, Savoca, Casteneda, Park, Esteban-Cruciani and Kalinec 12 Cisplatin blocks the calcium release during the depolarisation stage in the outer hair cells. The resulting increase in intracellular calcium can cause mitochondrial dysfunction. The ionic imbalance and disruption of intracellular mechanisms within the cell body subsequently results in cell death.Reference Sha, Taylor, Forge and Schacht 13
In 1978, Kemp demonstrated the production of acoustic energy by the outer cochlear hair cells, and termed this energy ‘otoacoustic emissions’.Reference Kemp 14 The energy that comes from the outer hair cells spreads through the base of the stapes, ossicles, tympanic membrane and outer-ear canal.Reference Prieve, Fitzgerald, Otoacoustic, Katz, Medwetsky, Burkard and Hood 15 This acoustic energy can be measured using a sensitive microphone placed in the ear canal. There are different types of otoacoustic emissions (OAE), but distortion product OAE (DPOAE) technology has good frequency specificity, and has been widely used in experimental studies.Reference Lonsbury-Martin, Martin, Telischi, Musiek and Rintelmann 16
Ginkgo biloba extract is composed of active substances, including 22–27 per cent flavonoids and 5–7 per cent terpenoids. This extract increases peripheral and cerebral blood flow, stimulates the production of antioxidant substances, reduces free oxygen radicals, and prevents cell damage associated with intracellular calcium increases.Reference Liu, Wu, He and Liu 17 – Reference Gulec, Iraz, Yilmaz, Ozyurt and Temel 19 It also inhibits lipid peroxidation reactions and enhances antioxidant enzyme activity.Reference Liu, Wu, He and Liu 17 – Reference Gulec, Iraz, Yilmaz, Ozyurt and Temel 19
This study aimed to determine the effectiveness of G biloba extract as an otoprotective agent against cisplatin-induced ototoxicity in rats.
Materials and methods
Animals and groups
The study protocol was approved by the Institutional Animal Care and Use Committee at Ondokuz Mayis University Medical Center, and the animals were treated in accordance with protocols approved by this committee. Twenty adult female albino Wistar rats were used in this study (weighing 180–230 g). All rats had free access to commercial food and water, and were maintained in an environment with controlled temperature (25°C) and 12-hour light/dark cycles. All rats were evaluated by otoscopic examination prior to distortion product OAE (DPOAE) testing. Rats with cerumen in the ear canal and/or tympanic membrane and/or middle ear problems were excluded from the study.
The animals with normal hearing (confirmed by DPOAE testing) were divided into two groups. In group one, 10 rats received an intraperitoneal, 12 mg/kg, single dose of cisplatin. In group two, 10 rats received an intraperitoneal, 12 mg/kg, single dose of cisplatin and daily doses of intraperitoneal 100 mg/kg Gingko biloba extract for 10 days.
Study design
All rats were anaesthetised with ketamine hydrochloride (50 mg/kg intraperitoneal) and xylazine (10 mg/kg intraperitoneal). All animals were observed for 17 days. Distortion product OAE measurements were obtained for both ears at the following time points: prior to cisplatin administration (baseline recording), and on days 10 and 17.
Otoacoustic emission test and recording apparatus
Distortion product OAE measurements were made using the Smart OAE machine (IHS, Miami, Florida, USA). Calibration and probe placement control were performed automatically using the OAE machine. The ears of 20 rats were evaluated (40 ears in total). The intensity levels of the DPOAE measurements were recorded as L1 for the f1 frequency (65 dB SPL) and L2 for the f2 frequency (55 dB SPL). Distortion product OAEs were recorded for 30 seconds at the following frequencies: 1, 1.5, 2, 3, 4, 6 and 8 kHz. Frequencies lower than 1 kHz were not recorded because of the internal noise of the rats. Distortion production OAE testing was performed on days 0, 10 and 17. The results were evaluated as signal-to-noise ratio values and compared according to frequency and group.
Statistical analysis was performed using independent sample t-test and repeated-measures analysis of variance methods.
Results
Table I shows the results of the distortion product OAE (DPOAE) test recorded prior to cisplatin administration (for all 40 ears). There was no statistically significant difference between the baseline signal-to-noise ratio values of each group, for either ear.
Table I Pre-cisplatin dpoae results

Freq = frequency; SNR = signal-to-noise; SD = standard deviation
The DPOAE measurements were repeated on days 10 and 17 for both groups. The results are shown in Tables II and III. On days 10 and 17, the signal-to-noise ratio values showed no difference between the two groups for 1, 1.5 and 2 kHz frequencies for either ear. However, there was a statistically significant difference between the groups at 3, 4, 6 and 8 kHz frequencies for both ears. The results of the DPOAE test, before and after cisplatin administration, are shown in Figure 1 (group one) and Figure 2 (group two).

Fig. 1 Distortion product otoacoustic emission results before and after cisplatin administration for group one in: (a) the right ears, and (b) the left ears.
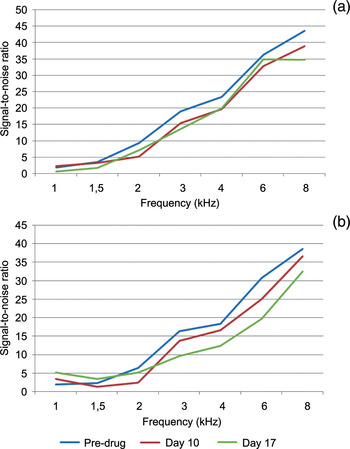
Fig. 2 Distortion product otoacoustic emission results before and after cisplatin administration for group two in: (a) the right ears, and (b) the left ears.
Table II Dpoae results on day 10

* p < 0.05. Freq = frequency; SNR = signal-to-noise; SD = standard deviation
Table III Dpoae results on day 17

* p < 0.05. Freq = frequency; SNR = signal-to-noise; SD = standard deviation
Discussion
The current study evaluated the efficacy of G biloba extract as an otoprotectant against cisplatin-induced ototoxicity in rats. Distortion product OAE (DPOAE) measurements were used to examine outer hair cell function. Comparisons of DPOAE measurements were made within and between groups. The results clearly showed that simultaneous administration of G biloba extract with cisplatin protected the outer hair cells and prevented cisplatin-induced hearing loss in rats.
Cisplatin has been shown to target three areas in the cochlea: the hair cells in the basal turn of the organ of Corti, the spiral ganglion cells and the lateral wall tissues (spiral ligament and stria vascularis). The outer hair cells, the cells in the stria vascularis and the cells in the spiral ligament have been shown to undergo apoptosis. In addition, platinated DNA immunoreactivity has been localised to the nuclei of outer hair cells, and cells in the stria vascularis and spiral ligament.Reference Rybak, Whitworth, Mukherjea and Ramkumar 10
In cisplatin-induced ototoxicity, hearing loss at high frequencies is generally reported 3–4 days following the first cisplatin dose. Hearing loss is usually accompanied by transient or permanent tinnitus.Reference Rybak, Clark and Ohlemiller 20 Cisplatin ototoxicity manifests as sensorineural hearing loss, which can be severe to profound following high-dose chemotherapy. Cisplatin-related hearing loss is usually bilateral and initially affects high frequencies.Reference Rybak and Rankumar 21 Pollera et al. found irreversible hearing loss following the use of 200 mg/m2 cisplatin for 5 days.Reference Pollera, Marolla, Nardi, Ameglio, Cozzo and Bevere 22 The authors found 15 dB or more hearing loss between 4–8 kHz in 75 per cent of patients who received two doses of the cisplatin treatment. Half of the patients in Pollera and colleagues' study showed 15 dB or more hearing loss after 48 hours following the first dose.
Cisplatin ototoxicity begins at the cellular level at 3 days and reaches its maximum level at 7–10 days; hence, DPOAE measurements were recorded on days 10 and 17 in the current study.Reference Lopez-Gonzalez, Guerrero, Rojas and Delgado 23 Compared with baseline values, the results for group one showed a significant decrease in DPOAE levels between 3–8 kHz on days 10 and 17. These findings confirmed the presence of cisplatin-induced hearing loss, consistent with the literature.
Cardinaal et al. found 65 per cent damage in the outer hair cells of rats following the administration of 12 and 16 mg/kg cisplatin.Reference Cardinaal, de Groot, Huizing, Veldman and Smoorenburg 24 They also found that cisplatin-induced ototoxicity increased with dosage, and that 12 mg/kg cisplatin dose was associated with the formation of hearing loss. Therefore, the present study used a dose of 12 mg/kg cisplatin to achieve hearing loss. In our study, no animal died using this dosage.
Several authors have investigated various agents for the prevention of cisplatin ototoxicity.Reference Choe, Chinosornvatana and Chang 4 , Reference Kalkanis, Whitworth and Rybak 6 , Reference Lopez-Gonzalez, Guerrero, Rojas and Delgado 23 , Reference Huang, Whitworth and Rybak 25 , Reference Meech, Campbell, Hughes and Rybak 26 Hyppolito et al. used amifostin to prevent cisplatin ototoxicity in guinea pigs.Reference Hyppolito, de Oliveire, Lessa and Rossato 5 The DPOAE measurements revealed no emission from the cisplatin group; however, emissions were observed in the cisplatin plus amifostin group after 3 days.
-
• Adverse effects of cisplatin include nausea, vomiting, neurotoxicity, nephrotoxicity and ototoxicity
-
• Cisplatin ototoxicity manifests as sensorineural hearing loss and tinnitus
-
• Some agents can ameliorate or prevent cisplatin-related hearing loss
-
• Ginkgo biloba extract is an otoprotective agent
-
• In this study, G biloba extract gave significant otoprotection against cisplatin-induced ototoxicity in rats
Kalcioglu et al. injected a single dose of cisplatin (16 mg/kg) into rats, and used erdosteine to prevent ototoxicity.Reference Kalcioglu, Kizilay, Gulec, Karatas, Iraz and Akyol 3 The findings for the cisplatin group showed a significant decrease in DPOAE levels between 3–8 kHz on day 5. However, there was no significant decrease for the cisplatin plus erdosteine group.
Antioxidants, such as D- or L-methionine, N-acetyl cysteine, sodium thiosulphate, lipoic acid, G biloba extract, aminoguanidine, alpha-tocopherol, ebselen combined with allopurinol, and salicylates, have been used to reduce cisplatin ototoxicity in animal experiments; presumably they act by scavenging the reactive oxygen species.Reference Rybak and Rankumar 21
Huang et al. investigated the effects of G biloba extract 761 on cisplatin ototoxicity.Reference Huang, Whitworth and Rybak 25 They examined group differences in auditory brainstem responses (ABR). Auditory brainstem responses were measured pre-treatment and 72 hours post-treatment, and threshold shifts were analysed. Cisplatin-treated rats showed significant ABR threshold shifts, but there were no significant threshold shifts in rats treated with G biloba extract 761 plus cisplatin. In addition, scanning electron microscopy indicated severe outer hair cell loss in the basal turn of the cochlea in cisplatin-treated rats; however, the outer hair cells remained intact in the rats treated with G biloba extract 761 plus cisplatin.
In the current study, ototoxicity was decreased in cisplatin-treated rats supplemented with G biloba extract. This supports the hypothesis that G biloba extract prevents outer hair cell death. Distortion product OAE measurements can be used to determine the early effects of ototoxic drugs that primarily affect the outer hair cells. Furthermore, DPOAE testing can be used in the early monitoring of cisplatin ototoxicity.







