Introduction
For patients with inner ear disorders, the most feasible and achievable routes of drug delivery are considered to be systemic and intratympanic administration. However, neither of these two drug delivery routes is perfect.
On the one hand, systemic administration (via the intravenous, intramuscular or oral route) cannot be used to treat many inner ear disorders because of the blood–labyrinth barrier, which limits the size and concentration of molecules from the arteriovenous circulation which can permeate into the inner ear.Reference Shi1 The undesirable systemic side effects arising from the high doses required to achieve therapeutic concentrations and durations within the inner ear limit the applicability of systemic administration in patients with conditions such as peptic ulcer, diabetes, hypertension and osteoporosis.
On the other hand, intratympanic administration, the most common topical method, is more likely to damage the anatomy and physiology of the tympanic membrane and/or eustachian tube, compared with systemic administration. The anatomical variability of the round window membrane implies the possibility of altered diffusion of intratympanically delivered particles through the round window and into the inner ear.
A third delivery route, intracochlear drug delivery has been investigated; however, this innovative method is not easy to perform as the cochlea is a closed space and lies deep within the temporal bone.Reference Borkholder, Zhu, Hyatt, Archilla, Livingston and Frisina2
Therefore, more suitable routes for drug delivery to the inner ear are needed.
Postauricular hypodermic injection has previously been used as a delivery route for local anaesthesia, for minor otological surgery, but has not previously been used as a treatment delivery method. Our group has previously investigated the use of glucocorticoid introduced into the postauricular space between the skin, the mastoid process and the posterior wall of the acoustic meatus, in the middle of the retroauricular groove, in volunteers with intractable, low-frequency, sensorineural hearing loss, with satisfactory therapeutic effects.Reference Yang, Yu and Ma3 In addition, this route of administration has been shown to achieve a higher concentration within the otocyst of guinea pigs, and a lower plasma concentration, compared with intramuscular injection.Reference Lin and Yu4 Thus, postauricular administration may represent a potential alternative drug delivery route, with the conceivable advantages of reasonable therapeutic effect, minimal invasiveness, simplicity, minimal damage to the middle ear, and relative freedom from influence by the blood–labyrinth barrier.
The current study investigated the applicability of postauricular administration of therapeutic agents to the inner ear by comparing perilymphatic pharmacokinetics following postauricular versus intravenous injection of gadopentetate dimeglumine, in an animal model. This was achieved using 7.0 Tesla magnetic resonance imaging (MRI), a noninvasive technique which enabled visual observation of the dynamic uptake of paramagnetic particles. Using this technique, we were able to assess whether postauricular administration resulted in satisfactory drug concentration and duration, within the inner ear.
Materials and methods
Animal preparation
The study used 12 male albino guinea pigs (300–400 g; Laboratory Animal Center, West China Hospital, Sichuan University, China), in accordance with the policies of the Beijing Ethics Committee on the Care and Use of Laboratory Animals. All animals were kept under standardised conditions, with constant humidity and temperature, a 12:12 hour day–night cycle, and free access to food and water.
The animals were randomly divided into two equal groups. Each of the animals was then administered gadopentetate dimeglumine (Schering, Berlin, Germany) via either postauricular or intravenous bolus injection, to a total dose of 3 ml/kg (0.5 mmol/ml). The postauricular delivery method and the total dose had both been previously tested in a pilot study.
Anaesthesia and drug delivery route
Animals were initially anaesthetised with an intraperitoneal injection of chloral hydrate (10 per cent, 3 mg/kg; Zhongke, Beijing, China), prior to gadopentetate dimeglumine delivery, and were then maintained on inhalational anaesthesia with isoflurane (1 per cent; Jiupai, Hebei, China), plus constant maintenance, during scanning. In the intervals between scanning, they were placed in an air-conditioned box supplied with fresh oxygen, water and food. Whilst anaesthetised, each animal's vital signs were monitored with a pulse oximeter (Surgivet, Waukesha, Wisconsin, USA). Rectal temperatures were kept within the physiological range (38.0 ± 0.5°C) using a thermistor-controlled, electrically heated pad (Elmedex Elektronic HB, Björklinge, Sweden).
Animals in the postauricular group were injected in the middle of the right retroauricular groove, releasing the gadopentetate dimeglumine solution into the postauricular space between the skin, the mastoid process and the posterior wall of the acoustic meatus.
Animals in the intravenous group were injected intravenously through the right femoral vein under sterile conditions.
Magnetic resonance imaging
The study used a Bruker Biospec USR animal MRI system (Bruker, Fällanden, Switzerland) with a magnetic field strength of 7.0 Tesla. This machine employed a self-shielded gradient system in combination with a semicircular, half-open, radio-frequency surface coil.
The animal was placed in the prone position with its head fixed in the centre of the magnetic field. The head position was adjusted so that both modioli were level. The image position and orientation of the cochleae for coronal T1-weighted MRI scanning were determined with the help of T2-weighted scanning in the sagittal and coronal planes.
For each scan, 30 high resolution, two-dimensional images with a slice thickness of 0.5 mm and a slice gap of 0.65 mm were acquired using the standard Bruker technique of spin echo sequencing. The scanning parameters were as follows: acquisition matrix, 256 × 256; and reconstruction matrix, 512 × 512.
Magnetic resonance imaging scans were taken before gadopentetate dimeglumine administration and then 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 24 and 48 hours afterwards.
In the MRI scans, inner ear fluid was originally bright in T2-weighted images (echo time/repetition time, 45 milliseconds/3000 milliseconds) and dark in T1-weighted images (echo time/repetition time, 14 milliseconds/673.2 milliseconds). The paramagnetic gadolinium ion reduced the relaxation lifetime of T1 scanning and enhanced the signal intensity after being absorbed.Reference Counter, Zou, Bjelke and Klason5
Image selection
Twenty-four coronal MRI scans were taken of each animal, just before and during the 48 hours after drug delivery. A total of 30 images, with a slice thickness of 0.5 mm, were acquired for each scan. Owing to the size of the inner ear, these imaging parameters produced approximately three images of the cochlea. The image that best showed the complete right modiolus was selected for further analysis.
Pharmacokinetics
Twelve images were chosen for each animal for pharmacokinetic analysis, one image for each time point.
The relative intensity of the scala tympani of the basal turn of the right cochlea was recorded as an indirect measure of dynamic gadolinium uptake into the perilymph, starting immediately before gadopentetate dimeglumine injection and ending 48 hours after injection. The pharmacokinetic parameters for gadopentetate dimeglumine uptake were determined using PKSolver software and utilising noncompartmental analysis.Reference Zhang, Huo, Zhou and Xie6 The following parameters were recorded: maximum intensity value of the T1 gadolinium-enhanced image; time to peak enhancement; elimination half-life; mean residence time; and area under the signal–time curve (from 0 to the last time point). The total dose (indirectly indicated by the total enhancement) was calculated from the product of signal intensity and time, i.e. the area under the signal–time curve. The maximum intensity value was theoretically directly proportional to the peak concentration of gadopentetate dimeglumine in the perilymph. The time to peak enhancement, elimination half-life and mean residence time for signal intensity were equivalent to similar parameters for drug concentration.
Statistics
The SPSS 13.0 software program was used for data analysis (SPSS Ltd, Chicago, Illinois, USA). One-way analysis of variance was used to determine the statistical significance of interactive effects among time factors and grouping factors. The independent, two-tailed Student's t-test was used to determine the statistical significance of results for the maximum intensity value, time to peak enhancement, elimination half-life, mean residence time and area under the signal–time curve. For each time point, number of data was six. The Kolmogorov–Smirnov test was used to analyse the distribution of samples. A two-tailed p value of less than 0.05 was considered statistically significant.
Results and analysis
The time course of gadolinium uptake into the perilymph was determined following either postauricular or intravenous injection. The 12 animals each received one type of administration.
Magnetic resonance images
Figure 1 shows temporal patterns for the gadolinium-enhanced, T1-weighted, right cochlear images. The general morphology of the four cochlear turns was initially indistinguishable in T1-weighted images, but was discernible in both the T2-weighted pre-administration images and the T1-weighted post-administration images with gadolinium contrast enhancement. This revealed a pattern of time-dependent gadolinium uptake increases and subsequent elimination, as indicated by signal enhancement and then signal reduction in the perilymphatic spaces of the scala tympani and scala vestibuli in each of the four turns. The most obvious changes were seen at the scala vestibuli of the basal turn and the scala tympani of the second turn, which was similar to previously reported observations.Reference Zou, Pyykkö, Counter, Klason, Bretlau and Bjelke7, Reference Mynatt, Hale, Gill, Plontke and Salt8 Although it was not the site of greatest or most rapid gadolinium uptake, the scala tympani of the basal turn showed the most homogeneous signal and the highest volume, of all the MRI scans analysed; this site was thus used for further observation.
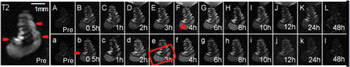
Fig. 1 Coronal magnetic resonance images of gadolinium uptake over time in the inner ear following postauricular administration (images A to L) and intravenous administration (images a to l). The time-dependent changes in signal intensity show a dynamic process of brightening (images A to E and a to d) to a peak (images F and e), followed by darkening (images G to L and f to l) to the original level. The rate of gadolinium uptake was comparatively greater in the lower turns (see red rectangle) than in the upper turns. Images of the scala media (arrowhead) in each turn were bright in T2-weighted images and dark in T1-weighted images, regardless of whether taken before (pre; images A and a) or after (images B to L and b to l) gadopentetate dimeglumine administration. The signal intensity of the scala tympani in the basal turn of the right cochlea (star) was measured for further comparison.
Similar time-dependent changes in gadolinium saturation (from pre-injection through the 48-hour post-injection period) revealed similar dynamic processes, i.e. a continuous increase to a peak followed by a decline, for both the postauricular and intravenous protocols. However, a much shorter process was observed for intravenous administration. Following intravenous injection, gadolinium enhancement had completely disappeared by 24 hours post-injection – a much shorter total duration compared with the postauricular route. The equilibrium phase lasted from the third to the eighth hour following postauricular delivery, but only from the first to the fourth hour following intravenous delivery (Figure 1).
It was also observed that images of the scala media (all turns) were bright in T2-weighted images and dark in T1-weighted images, both before and after gadopentetate dimeglumine administration, indicating that no obvious gadolinium uptake took place into the endolymphatic space.
Signal–time curves
Figure 2 shows time-course measurements obtained from the gadolinium-enhanced images, from pre-injection through to 48 hours post-injection, representing mean perilymph gadopentetate dimeglumine concentrations over time for both routes. The curve for animals receiving postauricular injection shows a slower, steadier process over the 48 hour post-injection period, while that for intravenous injection falls much earlier, and almost to the pre-injection level.
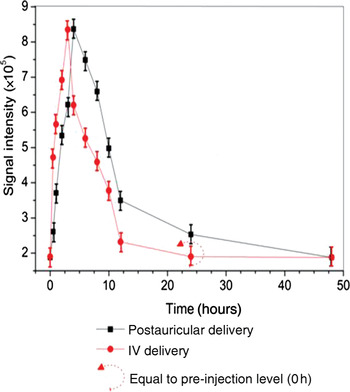
Fig. 2 Comparison of dynamic gadolinium uptake into the perilymph. Mean signal intensity was assessed from T1-weighted, gadolinium-enhanced magnetic resonance images. Animals receiving postauricular gadolinium had a slower, longer uptake and elimination, with a substantially delayed return to the original level, compared with animals receiving intravenous (IV) gadolinium.
Pharmacokinetics
Estimated pharmacokinetic parameters for gadopentetate dimeglumine in the right ear perilymph are summarised in Table I. Coefficients of variation denote individual variabilities. There was no statistically significant difference in maximum intensity value between the two delivery routes (p > 0.05). However, animals receiving postauricular injection showed a longer time to peak enhancement, longer elimination half-life and longer mean residence time, compared with animals receiving intravenous injection (p < 0.05) (Figure 3). There was also a significant difference between the two groups as regards area under the signal–time curve, with a greater area for the postauricular group compared with the intravenous group.
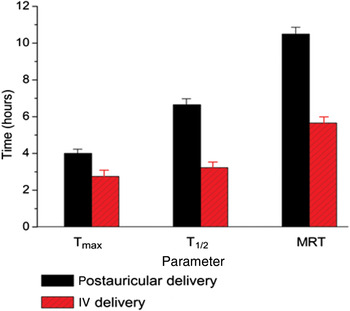
Fig. 3 Comparison of time-based pharmacokinetic parameters: time to peak enhancement (Tmax), elimination half-life (T1/2) and mean resident time (MRT). Significant differences were seen for all parameters, comparing animals receiving postauricular vs intravenous gadolinium administration.
Table I Pharmacokinetics of Gd-DTPA in perilymph*
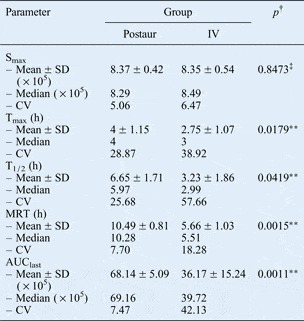
* In scala tympani of the basal turn of the right cochlea. †One-way analysis of variance. ‡No statistically significant difference, **statistically significant difference, for overall comparison of postauricular vs intravenous delivery. Gd-DTPA = gadopentetate dimeglumine; Postaur = postauricular; IV = intravenous; Smax=maximum intensity of T1-weighted, gadolinium-enhanced image; SD = standard deviation; CV = coefficient of variation; Tmax = time to peak enhancement; h = hours; T1/2 = elimination half-life; MRT = mean residence time; AUClast = area under signal–time curve from 0 to last time point.
As the curative effect of inner ear medication would be significantly influenced by the pharmacokinetic parameters equivalent to time to peak enhancement, elimination half-life and area under the signal–time curve, the results of the present study suggest that postauricular injection appears to increase the bioavailability of the injected agent, compared with intravenous injection.
Discussion
For patients with inner ear disorders, the most feasible and achievable routes of drug delivery are considered to be systemic and intratympanic administration. However, the systemic route sometimes cannot be used because of systemic side effects and difficulties with passage through the blood–labyrinth barrier. Furthermore, the intratympanic approach is not always reliable due to variability of the round window membrane.
Systemic drug delivery cannot be used to treat many inner ear disorders because of the blood–labyrinth barrier, which is similar to the blood–brain barrier, blood–eye barrier and blood–testis barrier in terms of anatomy, physiology and function. The blood–labyrinth barrier contains tight junctions between cells, which create a physical barrier limiting the size and electrical charge of molecules (including therapeutic agents) leaving the circulation and gaining access to the inner ear. Even if therapeutic agents can cross the blood–labyrinth barrier, they may be unable to attain a therapeutic concentration and duration in the inner ear without prolonged use of high systemic doses. Deleterious systemic side effects may result. For example, glucocorticoid is successfully used in the otological management of sudden sensorineural hearing loss, autoimmune inner ear disease and Ménière's disease, but its use is limited by undesirable side effects especially for patients with peptic ulceration, diabetes or hypertension.Reference Hamid and Trune9
Research on the round window membrane and the blood–labyrinth barrier has led to significant progress regarding the intratympanic application of drugs to the inner ear, potentially a much more effective delivery route. Nevertheless, intratympanic injection may cause mucosal injury of the tympanic membrane and eustachian tube, resulting in physiological imbalance and inflammation of the middle ear.Reference Juhn10, Reference Moskowitz, Lee and Smith11 In addition, the high level of variability of the round window membrane alters the degree to which this structure presents a physical barrier to the delivery of therapeutic substances to the inner ear.Reference Goycoolea12, Reference Mikulec, Hartsock and Salt13 For instance, Yoshioka et al. have reported that 13 per cent of human ears have poor round window membrane permeability, while 5 per cent have no permeability at all.Reference Yoshioka, Naganawa, Sone, Nakata, Teranishi and Nakashima14 In addition, Alzamil and Linthicum have reported the presence of round window niche obstruction in many human ears.Reference Alzamil and Linthicum15 Furthermore, drug loss via the eustachian tube compounds the degree of imprecision of intratympanic drug delivery.Reference Swan, Mescher, Sewell, Tao and Borenstein16, Reference Plontke, Mikulec and Salt17
Recent advances in cochlear prosthesis implantation and understanding of the molecular biology of hearing have prompted experimental investigation of intracochlear drug delivery. This delivery technique employs a cochleostomy in the surrounding bone or round window membrane, via which therapeutic agents can be directly perfused into the perilymphatic space, completely bypassing the blood–labyrinth barrier and round window membrane barrier. These intracochlearly delivered drugs are expected to reach their intended target in the optimal size and form, and always with precise dosage control. However, this delicate drug delivery technique has not yet been used clinically, except in the case of cochlear prosthesis implantation under general anaesthesia.Reference Salt and Plontke18
Postauricular hypodermic injection has been used to deliver local anaesthesia for minor otological operations, but has not previously been used for therapeutic purposes. Our previous research has indicated that this new method of drug delivery to the inner ear has the advantages of reasonable therapeutic effect, minimal invasiveness, simplicity, minimal damage to the middle ear, and relative freedom from influence by the blood–labyrinth barrier. When betamethasone was postauricularly injected to treat persistent, low-frequency, sensorineural deafness, treatment was effective in 82.6 per cent of patients and this effect was stable in the long term. In addition, dexamethasone has been noted to accumulate more readily in tissue homogenates of guinea pig otocysts when injected postauricularly, compared with intramuscular injection. These findings have been published in Chinese language journals.Reference Yang, Yu and Ma3, Reference Lin and Yu4, Reference Jing, Yu and Li19 They indicate that the postauricular approach is worthy of further study as a possible new route for inner ear drug delivery.
With the help of non-invasive MRI scanning, we were able to compare the effect of postauricular versus intravenous drug delivery on perilymph pharmacokinetics, visually and consistently.Reference Zou, Zhang, Poe, Qin, Fornara and Zhang20 Gadopentetate dimeglumine is a contrast agent widely used for clinical investigative imaging, and causes tissue and fluid to appear with heightened signal intensity on MRI scans. As gadopentetate dimeglumine does not disintegrate within the body, the signal intensity of a post-gadolinium-enhanced image will correspond to the concentration of gadopentetate dimeglumine in its constituent parts. Thus, in the present study enhancement of signal intensity in the perilymphatic space indicated time-dependent changes in gadolinium uptake, namely, a dynamic process involving a continuous increase to a peak followed by a decline. In contrast, there have also been reports that gadopentetate dimeglumine cannot permeate into the normal endolymph, to cause heightened signal intensity in the scala media, whether injected postauricularly or intravenously.Reference Counter, Bjelke, Borg, Klason, Chen and Duan21 This failure may possibly be due to the properties of the gadolinium-chelate compounds themselves, or to the inability of agents administered either postauricularly or systemically to penetrate the blood–labyrinth barrier.
The inner ear effects of any drug are influenced by the pharmacokinetic equivalents of time to peak enhancement, elimination half-life, mean residence time and area under the signal–time curve.Reference Plontke and Salt22 Thus, the results of the present study suggest that therapeutic agents administered via postauricular injection appear to be more bioavailable compared with those injected intravenously. In addition, our results support the hypothesis that postauricular injection may be more appropriate than systemic injection as a delivery route into the inner ear. Because there is no interaction between the in vivo contrast agent gadopentetate dimeglumine and the inner ear tissues, it is an effective tool with which to evaluate permeation and diffusion into the inner ear.Reference Lyford-Pike, Vogelheim, Chu, Della Santina and Carey23 Our findings provide a basis for visual observation of the distribution and pharmacokinetics of glucocorticoids in the inner ear, provided there is a successful coupling reaction between glucocorticoids and the gadolinium ion.Reference Lattuada and Gabellini24
In the current study, we observed a delayed time to peak enhancement, prolonged elimination half-life, extended mean residence time and greater area under the signal–time curve, for animals undergoing postauricular injection rather than intravenous injection. We propose the following three possible mechanisms for this observation (Figure 4).
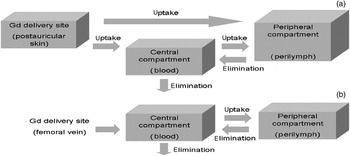
Fig. 4 Possible mechanisms of absorption and metabolism following (a) postauricular and (b) intravenous introduction of gadopentetate dimeglumine (Gd) into the blood and perilymph.
Firstly, transportation within the arteriovenous circulation may be an important and dominant mechanism. Gadopentetate dimeglumine could be absorbed into the circulation via postauricular capillaries and lymphatic capillaries and transported to the inner ear via its arterial supply. It is acknowledged that the absorption rate into the circulation following postauricular injection would be much slower than that following direct intravenous injection. If the gadopentetate dimeglumine in the perilymph comes primarily from the arteriovenous circulation, then postauricular application should lead to lower values for maximum intensity, area under the signal–time curve, time to peak enhancement, elimination half-life and mean residence time, compared with intravenous application. Our results contradict this hypothesis, strongly suggesting the presence of another main access route in addition to blood exchange.
• Postauricular injection may be a potential treatment route for inner ear disorders
• This route may avoid the systemic side effects of intravenous administration
• In this study, postauricular injection slowed the absorption and elimination of gadopentetate dimeglumine in the perilymph
• This agent cannot permeate into normal endolymph, whether postauricularly or intravenously injected
A second possible mechanism may involve various routes such as the round window membrane, oval window, tissue space and/or otocyst apertures. The osmotic pressure of injected, undiluted gadopentetate dimeglumine is seven times greater than that of normal extracellular fluid. Thus, as soon as it is infused into the postauricular space, gadopentetate dimeglumine will be continuously diluted by interstitial fluid and transudate from blood vessels and lymphatics. In this way, molecules of gadopentetate dimeglumine would diffuse from high permeability conditions to low permeability areas, including the middle ear and inner ear.Reference Takumida and Anniko25 This mechanism is congruent with the observed pharmacokinetic changes following postauricular injection.
The third, but most unlikely, mechanism involves perilymph derived from cerebrospinal fluid. On account of the blood–brain barrier, gadolinium is less likely to diffuse into the cerebrospinal fluid unless there is impairment of this barrier. Zou et al. noted that destruction of the cochlear aqueduct between the scala tympani and the cerebrospinal fluid did not reduce uptake of contrast agent into the perilymph.Reference Zou, Pyykkö, Counter, Klason, Bretlau and Bjelke7 Thus, the molecular basis of this possible mechanism remains to be determined.
Conclusion
This is the first study reporting the effectiveness of postauricular injection as a delivery route via which therapeutic agents could be introduced into the inner ear. This delivery route has the advantages of minimal invasiveness, simplicity, reasonable therapeutic effect, relative freedom from influence by the blood–labyrinth barrier, and minimal potential for damage to the middle ear. Thus, the postauricular approach warrants further research, and has potential as an alternative delivery route for the treatment of inner ear disorders.
Acknowledgements
We would like to thank Professor Xingqi Li of the Ear Institute at the General Hospital of People's Liberation Army for helpful comments, and Professor Guoliang Zhang of the Pharmacology Department at Peking University for patient guidance. We would also like to thank Yong Zhang of the China Pharmaceutical University for generous provision of the recently released PKSolver software program.
This study was supported by the National Natural Science Foundation of China (81070780) and the Capital Medical Development Scientific Research Foundation (20091015).







