Introduction
The petrous apex is the pyramidal-shaped medial projection of the petrous temporal bone. The apex sits within the angular interval produced by the greater wing of the sphenoid and the basilar part of the occipital bone forming part of the skull base. Anterolaterally to the petrous apex are the carotid canal, tensor tympani muscle and Eustachian tube. Posteriorly lies the internal auditory meatus and occipital bone with which it forms the jugular foramen. Superiorly within the apex is Meckel's cave, a depression for the trigeminal ganglion. Medial to the apex sits the clivus, and laterally the otic capsule. The petrous apex is made up of dense bone, bone marrow and, in those patients with pneumatisation, epithelium-lined air cells.
Pneumatisation is the development of air-filled spaces within bone due to epithelial infiltration during development,Reference Hill and Richtsmeier1,Reference Lee, Kim, Lee and Choi2 resulting in aerated spaces lined with mucus-secreting epithelia.Reference Hill and Richtsmeier1,Reference Virapongse, Sarwar, Bhimani, Sasaki and Shapiro3,Reference Kim, Song, Cho, Chang and Jun4 The pneumatisation of the temporal bone is initiated at 22–24 weeks' gestation, beginning with the mastoid antrum.Reference Diamant5,Reference Allam6 Aeration of the mastoid and remainder of the temporal bone continues after birth and ceases around puberty.Reference Kim, Song, Cho, Chang and Jun4–Reference Rubensohn7 Temporal bone pneumatisation is thought to arise primarily from positive pressures produced by the nasopharynx, transmitted through ventilation via the Eustachian tube.Reference Lee, Kim, Lee and Choi2,Reference Kim, Song, Cho, Chang and Jun4 Thus, the degree of temporal bone pneumatisation is suggested to be due to the degree of Eustachian tube ventilation.Reference Shinnabe, Hara, Hasegawa, Matsuzawa, Kanazawa and Kanazawa8 Pneumatisation is further contributed to by the extension of pneumatised mastoid antrum and mastoid air cells to the apex through multiple tracts around the otic capsule.Reference Lindsay9–Reference Curtin and Som11 These tracts have been described as supralabyrinthine, infralabyrinthine, posteromedial, anterior and along the arcuate artery.Reference Lindsay9
Aside from the mastoid process, pneumatisation of the temporal bone varies substantially between individuals.Reference Virapongse, Sarwar, Bhimani, Sasaki and Shapiro3,Reference Lindsay9,Reference Jen, Sanelli, Banthia, Victor and Selesnick12,Reference Schmalfuss13 In adults, aeration of the petrous apex is present in 11–40 per cent of the population and is regarded as a normal anatomical variant.Reference Lindsay9,Reference Grant, Welling, Oehler and Baujan14–Reference Cinamon16 The degree of pneumatisation varies between people, and, in the pneumatised petrous apices, 4–7 per cent are asymmetrical.Reference Virapongse, Sarwar, Bhimani, Sasaki and Shapiro3,Reference Roland, Meyerhoff, Judge and Mickey17,Reference Moore, Harnsberger, Shelton and Davidson18
The air tracts from the mastoid air cells into the petrous apex can provide pathways for infection and other disease in both children and adults.Reference Virapongse, Sarwar, Bhimani, Sasaki and Shapiro3,Reference Radhakrishnan, Son and Koch10,Reference Chapman, Shah, Curé and Bag19 Effusions in the petrous apex air cells can be idiopathic or occur in diseases like otitis media.Reference Moore, Harnsberger, Shelton and Davidson18,Reference Arriaga20 Despite resolution of the middle-ear disease, effusions can persist because of adhesive fibrosis of the communicating air tracts between the apex and middle ear.Reference Radhakrishnan, Son and Koch10,Reference Chapman, Shah, Curé and Bag19,Reference Arriaga20 Rarely, otomastoiditis can develop into apical petrositis (overt suppurative infection of the pneumatised petrous apex). Apical petrositis has the potential to cause osteomyelitis of the skull base and adjacent structures such as the meninges and cavernous sinus, which, although rare, can be fatal.Reference Wanna, Dharamsi, Moss, Bennett, Thompson and Haynes21 In addition, lesions such as cholesteatoma and cholesterol granuloma can occur in apex air cells.Reference Radhakrishnan, Son and Koch10,Reference Roland, Meyerhoff, Judge and Mickey17–Reference Chapman, Shah, Curé and Bag19 The presence of pneumatisation also has operative implications during procedures such as subtotal petrosectomy with blind sac closure, in which mucosa-lined air cells are obliterated to treat disorders including recalcitrant otitis media.Reference McKay-Davies, Selvarajah, Neeff and Sillars22
In summary, petrous apex pneumatisation is a normal anatomical variant, but has potentially significant implications for otological pathology and surgery. Although other aspects of the temporal bone have been widely studied, such as the volume of mastoid air cells, there has been minimal research investigating pneumatisation of the petrous apex, particularly using computed tomography (CT). This study aimed to investigate and quantify petrous apex pneumatisation in the paediatric population.
Materials and methods
Ethical approval was obtained from The Health and Disability Ethics Commission of New Zealand (approval code: 17/NTA/266), with institutional ethical approval from the Auckland District Health Board (approval code: A+7916).
Contrast-enhanced and non-contrast CT head scans of 1700 paediatric patients (aged 16 years or under) performed between May 2010 and December 2017 were acquired from Auckland City Hospital records. The axial and coronal CT scans were taken in 4 mm thick, contiguous, non-overlapping sections, and scanned using a 128-slice dual-source Somatom Plus 4 scanner (Siemens, Erlangen, Germany) as per Auckland City Hospital radiological protocols.23 Scans were taken from below the foramen magnum to the vertex of the skull. The most recent 100 scans from each 0–16 year group (0–1 years, 1–2 years, 2–3 years and so on) that met the inclusion criteria were reviewed by a single observer (TH) to assess for petrous apex pneumatisation.
In this population, reasons for the scans included trauma or intracranial assessment, and management of malignant, infectious, inflammatory or psychiatric diseases. Scans were excluded if they showed temporal bone trauma specifically or middle-ear disease, or if there was a record of this in their medical history based on diagnostic codes. Patients were also excluded if scans were ‘duplicate’ i.e. an individual patient had more than one scan. All patient scans were anonymised prior to image analysis.
The axial CT scans were analysed for prevalence of petrous apex pneumatisation in terms of: each year of age, whether petrous apex pneumatisation was unilateral or bilateral, and degree of petrous temporal bone pneumatisation (as a percentage volume). Sub-analysis based on gender and ethnicity was also carried out. Finally, the scans were assessed to examine communicating tracts between the petrous apex cells and the rest of the temporal bone.
Three slices were examined per patient scan: a slice at the level of the incudomalleolar joint, a slice above and a slice below (Figure 1). The incudomalleolar joint is easily identifiable and has been used in a previous study assessing pneumatisation in other parts of the temporal bone.Reference Park, Yoo and Lee24 In patients with bilateral pneumatisation, both sides were analysed. Computed tomography scan image analysis was carried out using ImageJ® Fiji® software.Reference Schindelin, Arganda-Carreras and Frise25 Techniques used to analyse air cell volume are similar to those previously described in mastoid pneumatisation.Reference Park, Yoo and Lee24,Reference Swarts, Doyle and Doyle26
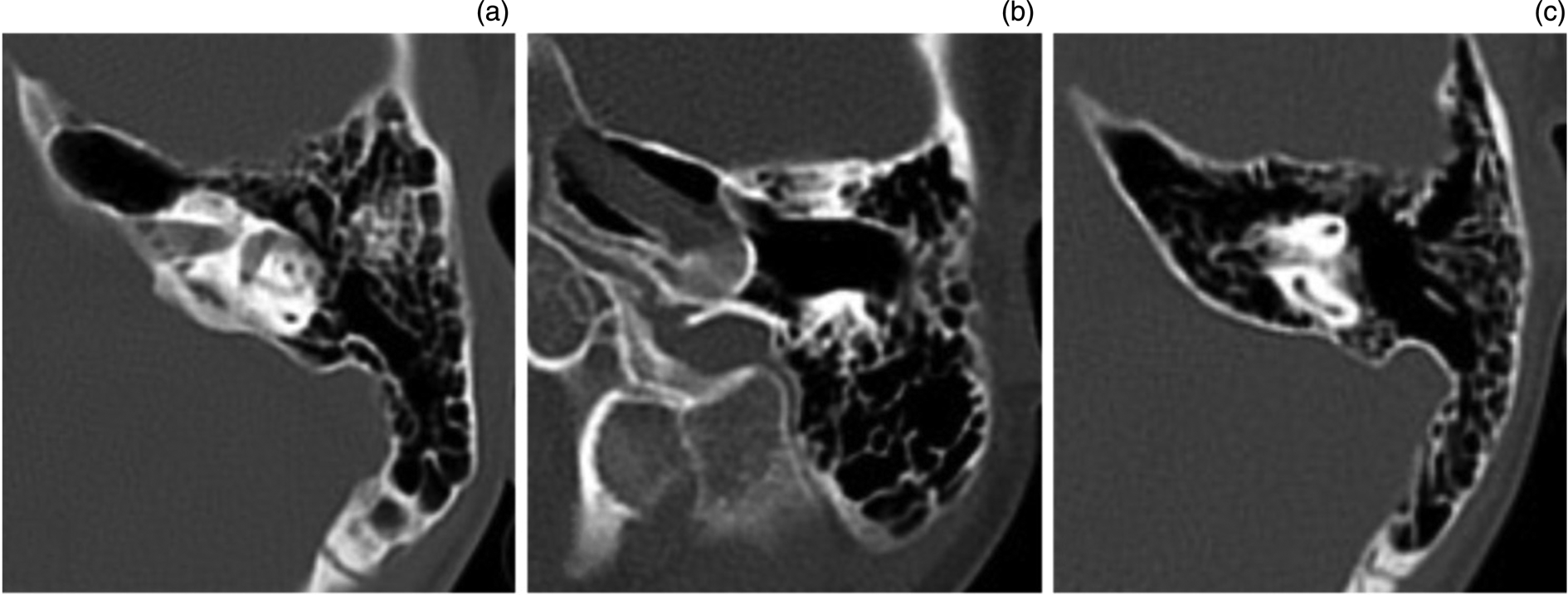
Fig. 1. Examples of petrous apex pneumatisation. (a) Petrous apex pneumatisation in the plane of the incudomalleolar joint, with communication with the mastoid antrum anterior to the otic capsule, in a 15-year-old patient. (b) Petrous apex pneumatisation in the inferior petrous apex at the level of the carotid artery in a five-year-old patient. (c) Petrous apex pneumatisation in the superior petrous apex, with superior and posterior semicircular canals visible, and communication with the mastoid air cell system, in a 10-year-old patient.
For each axial CT scan slice, the petrous apex was identified and, using a standardised identification method, isolated from the rest of the image using a cropping tool. Each image was converted to 8 bit. To isolate the aerated air spaces within the bone, the upper colour threshold levels of the image were altered between 65 and 115 (Figure 2). The area of air cells in each slice was measured using the ‘Measure’ tool in ImageJ software. The three slices of each scan were then used to calculate the volume of the air cells by multiplying the sum of the areas of each slice (in square centimetres) by the thickness of the slices (0.4 cm). The volume of the petrous apex bone was also calculated using this method, or manually traced around if threshold levels were not able to isolate the bone itself. The percentage of petrous apex pneumatisation in each patient was calculated by comparing the air cell volume with the total petrous apex bone volume.

Fig. 2. Example of image analysis using a computed tomography (CT) head scan. (a) Raw image of an axial CT head scan. (b) The isolated image of the petrous apex in 8-bit. (c) Air cells of the apex isolated by altering the image colour threshold.
Statistical analysis was carried out using SPSS software, version 25.0 (IBM, Armonk, New York, USA). The Spearman rank-order correlation test was employed to assess the strength of correlation between the prevalence of petrous apex pneumatisation and age. Chi-square tests were used to compare the prevalence of petrous apex pneumatisation between genders and ethnicities. Further Spearman rank-order correlations were used to determine the relationship between petrous apex pneumatisation volume relative to the petrous temporal bone and age, and for comparing volumes between genders.
Results
Overall prevalence
Of the 1700 scans studied, the prevalence of petrous apex pneumatisation in the study population was 21.0 per cent (357 out of 1700 patients). Of the patients with petrous apex pneumatisation, 50.4 per cent had unilateral pneumatisation and 49.6 per cent had bilateral pneumatisation.
Age data
The prevalence of pneumatisation for each age group can be seen in Figure 3. A Spearman's rank-order correlation determined that there was a very strong, positive relationship between age and the percentage of patients with pneumatisation, which was statistically significant (rs = 0.990, p < 0.001). No patients under the age of one year had petrous apex pneumatisation, while 38.0 per cent of 15-year-old patients had petrous apex pneumatisation.

Fig. 3. Percentage of patients with petrous apex pneumatisation at each year of age.
For those with pneumatisation, a Spearman's rank-order correlation was performed to determine the relationship between the age at which the scans were taken and the degree of petrous apex pneumatisation (as a percentage of total bone volume). There was a weak positive relationship between these variables, which was statistically significant (rs = 0.319, p < 0.001); this is reflected in Figure 4.

Fig. 4. Mean degree of petrous apex pneumatisation for each age group. Error bars show standard error of the mean.
Sex data
Males and females had a similar prevalence of petrous apex pneumatisation (21.4 per cent and 20.4 per cent respectively, Table 1). A chi-square test of independence found no significant association between the presence of pneumatisation and gender (χReference Lee, Kim, Lee and Choi2 (1) = 0.290, p = 0.591).
Table 1. Prevalence of petrous apex pneumatisation in male and female subgroups

Males and females also had a similar average degree of petrous apex pneumatisation in each age group. A Spearman's rank-order correlation found a weak positive correlation for both males (rs = 0.282, p < 0.001) and females (rs = 0.376, p < 0.001).
Ethnicity data
The ethnicity groups analysed were those used by the New Zealand census. The highest prevalence of petrous apex pneumatisation was found in the Latin American, Middle Eastern and African group, of 40.6 per cent; however, this group only contained 32 participants and contained a variety of ethnicities. All other groups had a prevalence of between 19 per cent and 25 per cent (Table 2).
Table 2. Prevalence of petrous apex pneumatisation in ethnic subgroups

A chi-square test of independence was used to compare the presence of pneumatisation for different ethnicities. There was a significant association between these variables (χ2 (4) = 10.631, p < 0.05). However, when the Latin American, Middle Eastern and African group was excluded, there was no significant association between the presence of petrous apex pneumatisation and ethnicity (χ2 (3) = 3.101, p = 0.376).
Communicating tracts
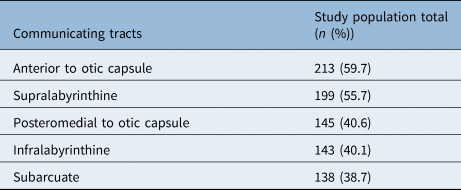
Communicating tracts were identified in 84.3 per cent of patients with petrous apex pneumatisation (301 out of 357) (Table 3). The most common tract identified was communicating with the mastoid system anterior to the otic capsule.
Table 3. Communicating tracts between petrous apex air cells and rest of aerated temporal bone
Discussion
Petrous apex pneumatisation in the paediatric population remains under studied. Previous studies analysing petrous apex pneumatisation have typically excluded children, resulting in a paucity of data on the epidemiology and development of the phenomenon.
• Petrous apex pneumatisation likely results from positive air pressures transmitted though Eustachian tube from the nasopharynx
• Communicating tracts between petrous air cells and the remaining middle-ear system may contribute to further pneumatisation
• Petrous apex pneumatisation prevalence was 21 per cent in this paediatric population; prevalence and degree of pneumatisation increased with age
• Sex and ethnicity did not affect degree or prevalence of petrous apex pneumatisation in children
• Communicating tracts were common when pneumatisation was present; the most prevalent configuration was anterior to otic capsule
• Petrous apex pneumatisation has diagnostic and procedural significance
The prevalence of 21.0 per cent was in keeping with studies of petrous apex pneumatisation prevalence in adults, which report prevalence values of between 11 per cent and 40 per cent.Reference Lindsay9,Reference Grant, Welling, Oehler and Baujan14–Reference Cinamon16 A previous investigation found evidence of apex pneumatisation in 3 per cent of their study population of 29 New Zealand children.Reference McKay-Davies, Selvarajah, Neeff and Sillars22 The difference in petrous apex pneumatisation prevalence between that study and our own may be due to variations in sample population and size.
When petrous apex pneumatisation occurs bilaterally, it does not appear to start symmetrically. Approximately half of the patients with petrous apex pneumatisation in our study population had unilateral air cells (49.6 per cent). In previous studies of adults, bilateral occurrence was more common; symmetrical pneumatisation with asymmetric patterns of aeration was a relatively rare occurrence at 4–7 per cent.Reference Virapongse, Sarwar, Bhimani, Sasaki and Shapiro3,Reference Roland, Meyerhoff, Judge and Mickey17,Reference Moore, Harnsberger, Shelton and Davidson18 The relative commonality of unilateral pneumatisation in this paediatric population compared to those of adults may be an indication that pneumatisation is not initiated at the same time bilaterally. Moreover, the process of pneumatisation initiation and development may be independent of the contralateral side.
Age
As children progress through childhood and into adolescence, they are more likely to have petrous apex pneumatisation, based on our findings. A previous study of 29 paediatric CT scans found evidence of pneumatisation occurring in a child as young as five years.Reference McKay-Davies, Selvarajah, Neeff and Sillars22 In our study, the youngest child with evidence of petrous apex pneumatisation was a one-year-old female, with 1 per cent of her petrous apices being pneumatised. The extent of pneumatisation was also found to vary with age in a study of 299 CT scans, with younger age being associated with greater pneumatisation; however, that study did not focus on children, with the median age of patients being 50 years (interquartile range, 33–63 years).Reference Tan, Ng, Lim, Low and Yuen27 It is unclear from these data why younger age was associated with greater pneumatisation in the adult population. Further investigation into petrous apex pneumatisation development later in life is warranted.
Unlike the mastoid air cell complex, in which pneumatised air cells are present at birth,Reference Diamant5,Reference Allam6 there was no evidence of petrous apex pneumatisation having been initiated pre-birth in our study population, as the youngest participant with petrous apex pneumatisation in this series was one year of age. It is difficult to draw conclusions on the typical age of onset of petrous apex pneumatisation in children; however, the prevalence increases throughout childhood. This is possibly a result of longer exposure to hypothesised contributing factors such as positive nasopharyngeal pressures, and invaginations of the middle meatus and mastoid complex, as discussed in previous studies.Reference Lee, Kim, Lee and Choi2,Reference Kim, Song, Cho, Chang and Jun4,Reference Radhakrishnan, Son and Koch10,Reference Curtin and Som11 The degree of pneumatisation does appear to become more varied with age, possibly reflecting varying ages of onset, rates and degrees of pneumatisation.
Of those patients with petrous apex pneumatisation, 84 per cent had some form of visible communicating tract connecting the air cells to the rest of the aerated temporal bone. Tracts have been found in as many as 86 per cent of adult temporal bones with petrous apex pneumatisation.Reference Lindsay9 Such tracts have also been observed previously in paediatric CT head scans.Reference McKay-Davies, Selvarajah, Neeff and Sillars22 In our study population, the most common communicating tracts were found anterior to the otic capsule. The presence of tracts communicating with the rest of the aerated temporal bone further highlights the hypothesised role of invagination of the petrous temporal bone in petrous apex pneumatisation formation and the potential implications of these tracts in the spread of disease.
Sex
A study evaluating the degree of petrous temporal bone aeration in 299 adult CT head scans using a subjective scoring system found a significant association between petrous apex pneumatisation and age and sex, with the male participants having significantly more extensive pneumatisation; however, the median age of participants in that study was 50 years.Reference Tan, Ng, Lim, Low and Yuen27 We found no significant difference in the prevalence or degree of petrous apex pneumatisation between males and females. As children reach full maturity, these differences between sexes may develop, but this is unclear from our case series.
Ethnicity
Petrous apicitis has been found to occur in 1 in 100 000 cases of otitis media in children.Reference Goldstein, Casselbrant, Bluestone and Kurs-Lasky28 In a New Zealand context, there are no published data on the incidence of the disorder except one case report involving an 11-year-old Maori female.Reference Rossor, Anderson, Steventon and Voss29 In New Zealand, Maori have a higher incidence of suppurative middle-ear disease when compared to other populations in New Zealand,Reference Johnston, McLaren, Mahadevan and Douglas30 with Maori and Pacific Islanders having higher rates of hospitalisation with acute otitis media.Reference McCallum, Craig, Whittaker and Baxter31 Previous studies have hypothesised poor aeration of the temporal bone as a risk factor for suppurative temporal bone disease,Reference Holmquist32 and petrous apex pneumatisation is the result of a well-ventilated otomastoid air cell system;Reference Shinnabe, Hara, Hasegawa, Matsuzawa, Kanazawa and Kanazawa8 however, we found the prevalence and extent of petrous apex aeration to be comparable between ethnic groups.
Clinical Relevance
Understanding the probability of petrous apex pneumatisation has both diagnostic and procedural significance. Diseases such as acute otitis media, cholesteatoma and cholesterol granuloma are unlikely to affect the petrous apex without pneumatisation.Reference Lindsay9,Reference Radhakrishnan, Son and Koch10,Reference Chapman, Shah, Curé and Bag19 This study suggests that a relatively high proportion of the paediatric population, over 20 per cent, may be at risk of petrous apex disease. Alternatively, if the patient is under one year of age, petrous apex disease should be lower down in the differential diagnosis. As the prevalence and extent of pneumatisation increases with age, patients in later childhood are at a higher risk of developing these diseases. Once the degree of pneumatisation is known, this knowledge affects surgical planning. For example, when performing a subtotal petrosectomy with blind sac closure, the degree of petrous apex pneumatisation affects the extent of temporal bone resection.Reference McKay-Davies, Selvarajah, Neeff and Sillars22
Limitations
High-resolution CT scanning is not typically used for neuroimaging in children in New Zealand because of radiation exposure, unless clinically indicated, often as a result of temporal bone or otological disease. The 4 mm thickness between slices meant an assumption had to be made that there was no variability in air cell area between slices that might affect the accuracy of the volume calculations. Rotation of the head in any plane could alter the air cell area seen in each slice, as is the case in any CT imaging. Nevertheless, these results have been assessed largely in relative terms, with an emphasis on the prevalence or degree of pneumatisation as opposed to absolute values. Furthermore, communicating tracts between petrous apex cells and the rest of the temporal bone may be more common in patients with petrous apex pneumatisation, and just not radiologically visible on the scans examined.
Structures inherently embedded in the petrous apex, such as the carotid artery in the inferior apex, were not accounted for in volume measurements, and thus contributed to the measurement of bone volume. This, however, would not have an effect on the proportion of the petrous apex that is pneumatised.
Finally, as the study participants had required a CT head scan to investigate intracranial pathology, the population may not reflect the healthy New Zealand paediatric population in its entirety.
Conclusion
As diseases of the petrous apex have previously been found to occur almost exclusively in pneumatised petrous temporal bones,Reference Lindsay9 there is clinical utility in understanding the patterns of petrous apex pneumatisation in the paediatric population. This is one of the largest case series of its kind. Petrous apex pneumatisation becomes increasingly prevalent and extensive throughout childhood and early adolescence. This is likely due to a variety of factors, including exposure to positive nasopharyngeal pressures and invaginations from air cells in the rest of the temporal bone throughout childhood. There does not appear to be a predominance based on sex or ethnicity. Communicating tracts are seen in the majority of paediatric patients with petrous apex pneumatisation, most commonly anterior to the otic capsule.
Competing interests
None declared









