Introduction
Attic cholesteatoma is a common type of acquired middle-ear cholesteatoma that develops from a retraction pocket in the relaxation part of the tympanic membrane.Reference Semaan and Megerian1 The traditional operation for attic cholesteatoma is microscopic surgery,Reference Charachon, Schmerber and Lavieille2 and endoscopy is mainly used for intra-operative middle-ear exploration.Reference Karchier, Niemczyk and Orlowski3–Reference Dedmon, Kozin and Lee5 However, with the development of endoscopy in recent years, the operation for cholesteatoma in the middle ear and upper tympanic cavity under transcanal endoscopy has been carried out gradually, and it has the same effect as microscopic surgery in terms of hearing recovery and recurrence rate. In addition, endoscopic ear surgery has the characteristics of a wide field of vision, minimal trauma and a short operation time.Reference Magliulo and Iannella6–Reference Alicandri-Ciufelli, Marchioni, Kakehata, Presutti and Villari10
However, there are limitations of endoscopic ear surgery including:Reference Bottrill, Perrault and Poe11,Reference Kozin, Lehmann, Carter, Hight, Cohen and Nakajima12 (1) for novice surgeons, the one-handed operation is time-consuming and unstable. Therefore, skilled endoscopy operation experience and long-term training are required; (2) the depth and stereo perception of the surgical field under endoscopy are poor. Planar vision results in some difficulty for beginners; (3) the operation space of the external auditory canal is small, and when endoscopy is performed inside the canal, it is easy to touch the wall and cause bleeding. At the same time, the mirror surface in endoscopy is easily blurred by blood at high temperature, so the lens needs to be wiped repeatedly; and (4) the thermal effect of the light source may cause damage. During the operation, long-term endoscopic exposure of the promontory should be avoided to prevent damage to the cochlea.
Bone drilling is the most difficult and time-consuming procedure in endoscopic surgery for attic cholesteatoma. While maintaining a clear view, it is also necessary to complete the procedures of drilling, flushing and cleaning the bone powder with one hand in a narrow space. The procedure can be performed with both hands under microscopy, so there is no problem with continuous bone drilling. However, the operator can only use one hand, which leads to step-by-step drilling of the bone, thus greatly prolonging the operation time. We repeatedly considered these shortcomings of the one-hand operation during the procedure and found that underwater continuous drilling with a pressure infusor was a good way to reduce the difficulty of the operation. This paper compares the differences between underwater continuous drilling with a pressure infusor and traditional intermittent drilling with a syringe in terms of the operation and anaesthesia times, the prognosis, and hearing improvements for attic cholesteatoma.
Materials and methods
General data
A retrospective analysis of patients with upper tympanic cholesteatoma who underwent transcanal endoscopic ear surgery from January 2015 to December 2017 was carried out. Patients were randomly assigned to two groups. Forty patients underwent underwater continuous drilling (group A), and twenty-one patients underwent traditional intermittent drilling (group B).
The inclusion criteria were as follows: patients over 18 years of age; patients with middle-ear cholesteatoma confined to the epitympanum; and patients undergoing partial ossicular replacement prosthesis implantation. The post-operative follow-up time reached six months. All patients underwent ear surgery for the first time.
The exclusion criteria were as follows: poor physical condition; external auditory canal malformation such as stenosis; cholesteatoma involving the mastoid antrum and cavity and invasion of the stapes, semicircular canal, and facial nerve; patients without ossiculoplasty; patients with total ossicular replacement prosthesis implantation; and patients whose pure tone threshold, acoustic immittance and ear computed tomography (axial bone scan magnification and coronal reconstruction) examinations were performed before surgery.
Except for the inconsistent methods of atticotomy, the other surgical procedures were consistent. All the tympanic membranes were repaired with tragal perichondrium autografts. All tympanic cavities and external auditory canals were filled with gelatin sponges. The patients in the two groups were followed for the first week, first month, second month, third month and sixth month after surgery, and the total follow-up time was six months. Follow up included the observation of tympanic membrane growth, pure tone audiometry and the evaluation of complications.
Equipment and instruments
The German Storz (Tuttlingen, Germany) rigid tube endoscopic set, with an external diameter of 3.0 mm, angles of 0° and 30°, and a length of 14 cm was used with a cold light source and an endoscopic imaging system. A set of conventional ear microsurgery instruments and a Bien-Air (Biel/Bienne, Switzerland) electric drill in the otological power system with a 2.0–4.0 mm diamond bur head were used.
Operating room layout
The patient was in the supine position with the affected ear inclined upward at 45 degrees, and the operator was sitting on the side of the affected ear. The trolley was equipped with a cold light source, display and video recording system, which was placed on the opposite side of the affected ear, approximately 1.5 meters in front of the operator. The operating table was placed on the side close to the patient's head, the nurse providing instruments and the surgeon were seated side by side, the suction device was placed on the right-hand side of the operator, and the ear power system and the pressure infusor flushing device were located at the end of the patient's non-operative side near the bed.
Underwater continuous drilling with a pressure infusor
A disposable, sterile special connection plastic pipe was connected to the water injection hole of the electric drill, the middle pipe was placed in the tank of the liquid pump pressurising device, and the other end was connected to a warm plastic package for an injection of 500 ml of 0.9 per cent sodium chloride. There were two start-up modes: (1) the otological electric drill and the flushing of the pressure infusor ran simultaneously, and (2) the otological electric drill was not synchronised with the pressure infusor, and pressure flushing was completed only when the foot tread reached the bottom.
During the operation, the synchronous operation mode was adopted, and there were 10 pre-set flow rates. The growth rate of each file was 15 ml/minute. We used 150 ml/minute during the operation. Guided endoscopy was used to place the electric drill into the middle-ear cavity, and the starting device was pressurised. Water was washed into the cavity, and blood was stained. When vision was clear, the drill was continuously powered to remove the bone under water. The endoscope was continuously placed in the water. It did not need to be wiped out. A 3.2 mm straight cutting bit was used for continuous underwater drilling at 40 000 rpm (Figure 1 and 2).

Fig. 1. Clear underwater drilling field of vision. (a) Shows the cavity filling with water, (b) shows the surgical cavity before underwater drilling, and (c) shows the surgical cavity after underwater drilling.

Fig. 2. Sketch of underwater drilling (the cavity was full of water).
During the operation, sufficient water pressure dislodged the bone powder and quickly removed it from the ear canal. At the same time, a large amount of flowing water continued to wash the mirror surface to ensure clear underwater vision. According to the underwater clarity, the water pressure was adjusted. If there was too much tissue debris during the operation, the underwater visibility was reduced, and the water pressure irrigation cavity could be increased to continue the underwater operation after the vision was clear.
During the operation, the endoscope was always in the water, and there was no need to withdraw the lens of the endoscope and suck out the residual irrigation fluid in the middle-ear cavity at different times. It should be noted that the infusion pipe connecting the pump should not be too long, as this may cause insufficient water pressure and affect underwater clarity. Plastic sheets were placed in the operative cavity to protect the flaps and avoid tissue damage by the underwater electric drills.
Traditional intermittent drilling with a syringe
A 20 ml syringe was equipped with 0.9 per cent sodium chloride at room temperature and a 5 ml syringe needle, and the needle was bent to 30°. The drill power system was started, with a 3.2 mm straight cutting bit at 4000 rpm. The assistant held the 20 ml syringe and dripped water slowly along the electric drill at the external ear canal mouth to keep the polished bone powder moist and to keep it from splashing. The assistant adjusted the dripping speed according to the drilling speed of the electric drill and stopped the operation when the bone powder floated and blocked the operation area and field of vision. The endoscope was then removed, the lens was wiped, the bone powder in the middle-ear cavity was washed with a large amount of room-temperature saline, and the residual washing liquid was removed with a tympanum aspirator. This method exposed the upper tympanum intermittently.
Hearing test
A pure-tone audiometer was used to evaluate the hearing level of patients before and after the operation. According to the standard test scheme, patients were tested in the room without environmental noise. Threshold values of air and bone conduction were calculated at 500, 1000 and 2000 Hz. The above frequencies represent the range of speech frequencies, and an increase in these frequency thresholds has clinical significance. The air–bone gap (ABG) was calculated by subtracting the bone conduction threshold from the air conduction threshold. Post-operative ABG less than 20 dB HL indicated hearing improvement.
Statistical methods
Statistical processing was completed using SPSS® (version 23) statistical software. Enumeration data were described as the number and percentage of cases, and the chi-square test or Fisher's exact test was used. The measurement data were described as the mean value ± standard deviation, and a t-test was used. Statistical significance was set at p < 0.05.
Results
Comparing patient information
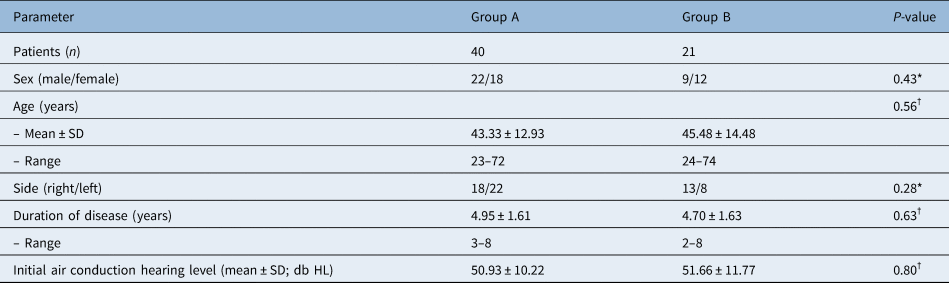
Table 1 summarises the basic information of the patients in the two groups. There were no significant differences between the two groups in terms of age, sex, side, duration of disease or initial air conduction.
Table 1. Demographics and baseline characteristics of the patients in the two groups

*Chi-square test; †independent-sample t-test; SD = standard deviation.
Comparing intra-operative conditions
The data from each group were normally distributed. The operation time was 64.61 ± 12.90 minutes in group A and 79.60 ± 16.81 minutes in group B (p = 0.025). The anaesthesia time was 102.69 ± 17.93 minutes in group A and 119.82 ± 19.28 minutes in group B (p = 0.019; Table 2).
Table 2. Operation time and anaesthesia time in groups A and B

*Independent-sample t-test; SD = standard deviation.
Comparing post-operative conditions
The dry ear time was more than 1 month: there were 38 ears (96 per cent) in group A and 20 ears (94 per cent) in group B, and there was no significant difference (p = 1.000). Six months after the operation, the average air conduction threshold of group A was increased by 20.13 ± 2.54 dB HL, while that of group B was increased by 21.09 ± 1.98 dB HL, with no significant difference (p = 0.144).
One patient suffered from injury to the semicircular canal, which occurred in group B. The patient developed vertigo after the operation and improved after one week of symptomatic treatment. During the operation, the chorda tympani nerves were severed in two patients: one in group A and one in group B. All of the nerves were severed when the granulation of the tympanum was cleaned with the circumferential knife and were not injured by the electric drill (Table 3).
Table 3. Comparison of post-operative data between group A and group B

* = Independent-sample t-test; † = chi square test; ‡ = Fisher
Discussion
With the rapid development of endoscopic technique in recent years, its use for middle-ear cholesteatoma has also shown advantages.Reference Ayache, Tramier and Strunski13 Endoscopy brings minimally invasive and precise technology into otology, changes the concept of surgery, and provides a new understanding of the anatomy and physiology of the middle ear.
Studies have shown that there are no significant differences in the incidence of recurrence and residual lesions between endoscopic surgery and traditional microscopic surgery.Reference Alicandri-Ciufelli, Marchioni, Kakehata, Presutti and Villari10,Reference Presutti, Gioacchini, Alicandri-Ciufelli, Villari and Marchioni14,Reference Glikson, Yousovich, Mansour, Wolf, Migirov and Shapira15 Residual cholesteatoma of the middle ear is the main factor that leads to surgical failure. The facial recess, anterior superior tympanic recess and tympanic ostium of Eustachian tube are the main locations of recurrence.Reference Marchioni, Alicandri-Ciufelli, Molteni, Artioli, Genovese and Presutti16
Based on the indications for appropriate surgical selection, endoscopic treatment for cholesteatoma in the upper tympanum can reduce residual lesions. Under multiangle endoscopy, the field of vision can be explored without a dead angle in the tympanum; however, in the process of removing lesions, this approach does not meet the requirements of visual accessibility. In addition, each tympanum of the middle-ear cavity is hidden in the bone. During the operation, it is often necessary to remove the exposure field of bone on the lateral wall to thoroughly remove the disease and minimise recurrence and residual probability.Reference Marchioni, Alicandri-Ciufelli, Piccinini, Genovese and Presutti17–Reference Thomassin, Korchia and Doris19
Bone drilling is the most difficult part of endoscopic surgery. The surgeon cannot complete the electric drilling, bone drilling, flushing and bone powder cleaning with one hand. Under the microscope, a hand-held electric drill with a water inlet and a hand-held aspirator are often used to drill bones. However, this approach cannot be used in endoscopic surgery. Some doctors use a small amount of water to drill bone, but there is no way to absorb the liquid and the bone powder in time. After a few seconds, they cannot see the structure under the drill bit. As a result, they need to stop the electric drill frequently, clean up the bone powder and liquid and then drill again. This time-consuming approach is obviously prolonged, making endoscopic bone drilling one of the greatest difficulties in the development of endoscopic surgery.
Our underwater drilling approach is different from that described by Yamauchi et al.Reference Yamauchi, Yamazaki, Ohta, Kadowaki, Nomura and Hidaka20 in 2014, as they achieved underwater drilling by injecting water into the cavity. At the same time, our underwater drilling approach is also different from that described by Yamauchi et al.Reference Yamauchi, Hara, Hidaka, Kawase and Katori21 in 2017 and Nishiike et al.Reference Nishiike, Oshima, Imai and Uetsuka22 in 2019, as they achieved underwater drilling by scrubbing and cleaning the lens under endoscopy. To achieve underwater drilling, we used a common otological drill with a water irrigation hole that could be connected to the pressure infusor. We excluded patients without ossiculoplasty because the lesions of these patients were generally mild, and there might be no significant hearing loss in these patients before the operation. This paper compared the hearing improvement rate of the two groups, with the study aimed at patients with hearing loss before operation. Patients with total ossicular replacement prosthesis implantation were excluded because their diseases were often serious, and cholesteatomas were not only limited to the upper tympanic cavity.
The operation time was 64.61 ± 12.90 minutes in group A and 79.60 ± 16.81 minutes in group B (p = 0.025). The anaesthesia time was 102.69 ± 17.93 minutes in group A and 119.82 ± 19.28 minutes in group B (p = 0.019). There was no significant difference between the two groups in terms of the improvement in the air conduction hearing threshold, dry ear time, non-healing perforation rate, hearing improvement rate or post-operative complications. This shows that underwater drilling is a valuable surgical method.
During the operation, a hand-held endoscope and another hand-held electric drill were used. The water in the operation cavity immediately flushed the dislodged bone powder and tissue fragments out of the ear canal keeping the operation field clean and overcoming the disadvantages of one-handed operation. At the same time, the flowing water washed the endoscope lens against the current to obtain a clear field of view and to ensure that the endoscope continued to work underwater. The pressurised underwater operation ensured the cavity always had low-temperature flowing water, which is conducive to haemostasis, while minimising the thermal damage of the light source and electric drill to the tissues and also avoiding the production of fog on the lens.
• Underwater continuous drilling is easy to achieve
• The operation times were shorter in the underwater continuous drilling group
• The anaesthesia times were shorter in the underwater continuous drilling group
• There was no difference in surgical outcome and hearing recovery between the two groups
Endoscopic surgery is becoming increasingly widespread, and its advantages are obvious, although it requires the operator to master complex ear anatomy and pathophysiology knowledge. Endoscopy, as a surgical tool, is in the initial stages of development. It is necessary to continue to explore the use of instruments to improve advantages and avoid disadvantages. In clinical practice, we should make a reasonable operative plan and choose the right tools according to the characteristics of the lesions to achieve the best therapeutic effect. Underwater continuous drilling with a pressure infusor in endoscopic surgery can help obtain a clear surgical field to reduce operation difficulty. This method is time-saving and labour-saving during surgery, avoids many disadvantages of the single-handed operation and is conducive to retaining structure or function. The device is simple and convenient for clinical use and is thus worth trying.
Conclusion
The results showed that the operation time and anaesthesia time were shorter in group A than in group B, but underwater continuous drilling with the pressure infusor achieved the same good outcomes as traditional intermittent drilling with a syringe for attic cholesteatoma. However, we still need further prospective studies on the subjective and objective factors, results and theoretical basis of the two groups to better understand the effect and role of underwater continuous drilling with a pressure infusor in otology operations.
Acknowledgement
This study was supported by National Natural Science Foundation (No. 81670933).
Competing interests
None declared