Introduction
Olfactory neuroblastoma (esthesioneuroblastoma) is a rare tumour arising from olfactory epithelium, and accounts for approximately 3–5 per cent of all sinonasal malignancies.Reference Broich, Pagliari and Ottaviani 1 Approximately 1400 cases have been reported in the literature since 1924, when the condition was first described by Berger and Luc.Reference Petruzzelli, Howell, Pederson, Origitano, Byrne and Munoz 2 There is no recognised underlying cause or genetic predisposition.
Patients typically present late with locally advanced disease, often with up to a one-year history of non-specific symptoms, most commonly nasal obstruction, epistaxis and hyposmia. Erosion of the paranasal sinuses, orbit and/or skull base is a common feature at presentation. Regional and metastatic dissemination occurs, with 8–20 per cent of patients having clinical or occult cervical nodal involvement at presentation.Reference Rimmer, Lund, Beale, Wei and Howard 3
Histologically, olfactory neuroblastoma bears close resemblance to other round cell neuroendocrine tumours and, given differences in propensity for spread, accurate tissue diagnosis is essential. Immunohistochemistry, particularly S-100 stain, can be valuable in distinguishing sinonasal undifferentiated carcinoma and sinonasal neuroendocrine carcinoma from classically S-100 positive olfactory neuroblastoma.
Beyond histological diagnosis, Hyams grading system differentiates low (1–2) and high (3–4) grade tumours.Reference Saade, Hanna and Bell 4 Pathological features considered include: tumour architecture, cellular pleomorphism, mitotic activity, presence of necrosis or calcifications, and presence of neurofibrillary matrix or rosettes.
Clinical staging has evolved in an attempt to prognosticate and stratify treatment approaches. The most widespread classification system in use was first proposed by Kadish et al. in 1976.Reference Kadish, Goodman and Wang 5 These authors distinguished tumours limited to the nasal cavity from those invading the paranasal sinuses and those extending beyond the nasal cavity and sinuses, into groups A, B and C, respectively. Morita et al. later modified this system (in 1993) with the addition of stage D, which accounted for patients who had distant spread of disease.Reference Morita, Ebersold, Olsen, Foote, Lewis and Quast 6 In 1992, Dulguerov and Calcaterra proposed a tumour–node–metastasis (TNM) staging system, which incorporated pre-operative computed tomography (CT) and magnetic resonance imaging (MRI) findings.Reference Dulguerov and Calcaterra 7
There is no clearly defined treatment protocol for this disease, and no large prospective studies have ever been conducted. The acceptance of open craniofacial resection as a viable surgical option in the 1970s led to a documented significant improvement in patient outcomes compared to previous extracranial resection.Reference Krischek, Godoy, Zadeh and Gentili 8 Studies have consistently demonstrated a survival benefit of adjuvant radiotherapy in patients with locally advanced tumours.Reference Chao, Kaplan, Simpson, Haughey, Spector and Sessions 9 , Reference Eich, Hero, Staar, Micke, Seegenschmiedt and Mattke 10 Most recently, with the advent of endoscopic technologies, use of a transnasal surgical approach has achieved comparable outcomes to traditional open craniofacial resection.Reference Devaiah and Andreoli 11 – Reference Fu, Monteiro, Muhanna, Goldstein and de Almeida 13 With olfactory neuroblastoma known to be both chemosensitive and radiosensitive, variable applications of combined chemoradiotherapy have been evaluated, with mixed outcomes.Reference Bak and Wein 12 , Reference Polin, Sheehan, Chenelle, Munoz, Larner and Phillips 14 The majority of chemotherapy regimens are platinum-based.
The role for elective treatment of cervical nodal basins remains particularly contentious. The overall incidence of cervical nodal metastasis, including at first presentation or subsequently during follow up, is up to 33 per cent in some series.Reference Petruzzelli, Howell, Pederson, Origitano, Byrne and Munoz 2 , Reference Zafereo, Fakhri, Prayson, Batra, Lee and Lanza 15 Recurrence can occur late and is most common at the primary site or the cervical lymph nodes.Reference Rimmer, Lund, Beale, Wei and Howard 3 Given the often protracted time course and unpredictable nature of recurrence, long-term clinical and radiological follow up has previously been recommended.Reference Rimmer, Lund, Beale, Wei and Howard 3
In this study, we report the experience of a multidisciplinary head and neck cancer unit over a 14-year period, with a focus on treatment approach and the role of long-term surveillance. In retrospectively evaluating management at a tertiary centre, we aimed to inform future treatment and follow-up protocols. We also aimed to provide insight into the natural history of this rare condition, from diagnosis through to post-treatment.
Materials and methods
This is a retrospective review of patients with histologically proven olfactory neuroblastoma treated at the Princess Alexandra Hospital from 2000 to 2014. Institutional ethics approval was obtained from the Metro South Human Research Ethics Committee.
Only patients with histologically confirmed olfactory neuroblastoma treated with curative intent were included in this study. All patients underwent imaging studies to define disease extent prior to intervention. Demographic and clinicopathological data were extracted from a prospective database, and were cross-correlated with hospital patient medical records and laboratory data. Fields included: sex, age, presenting symptoms, date of primary diagnosis, Hyams grade, Kadish stage, TNM stage, presence of nodal or distant disease, surgical and adjuvant radiotherapy and/or chemotherapy treatments, relapse status, and status at last follow up. Relapse was defined as the first instance of confirmed disease recurrence. Locoregional relapse was defined as recurrence in the head and neck. Data on adverse events were also sought, specifically the occurrence of cerebrospinal fluid (CSF) leak or post-operative collection, and whether radiological or operative intervention was required.
Data were de-identified post-collection. Statistical analysis was conducted using GraphPad Prism software, version 6.04 for Windows (GraphPad Software, La Jolla, California, USA). Endpoints for analysis included disease-free survival and overall survival. Survival was calculated from the start of primary treatment to the date of relapse, death or last follow up, as appropriate. Survival curves were generated using the Kaplan–Meier method.
Results
Over the 14-year period, 11 patients were treated at our institution for olfactory neuroblastoma with curative intent. Seven patients (64 per cent) were female and 4 (37 per cent) were male, with a median age of 61 years (range, 46–85 years).
The majority of patients (64 per cent) presented with more than one symptom, the most common being unilateral nasal obstruction (64 per cent), anosmia or hyposmia (45 per cent), epistaxis (45 per cent), and rhinorrhoea (27 per cent). One patient presented with headaches, epiphora and rhinorrhoea.
At presentation, the tumour was confined to the nasal cavity (Kadish stage A) in one patient (9 per cent), the tumour involved the nasal cavity and paranasal sinuses (Kadish stage B) in one patient (9 per cent), and the tumour extended beyond the paranasal sinuses (Kadish stage C) in eight patients (72 per cent). One patient (9 per cent) had distant disease with a thyroid metastasis at presentation (Kadish stage D). Regarding TNM staging of the primary tumour, seven patients had T4 disease, three patients had T3 disease and one patient had T1 disease. No patients presented with primary nodal disease and only the one patient had distant metastasis. Four patients had low-grade disease and three patients had high-grade disease on histopathology by Hyams grading system; four patients were not assessed.
Treatment
In the patient with Kadish stage A disease, the tumour involved the left nasolacrimal duct on pre-operative imaging. This individual underwent endoscopic-assisted wide local excision, including medial maxillectomy. No adjuvant therapy was given and the patient remains alive, with no evidence of disease at the last follow up (40 months). The patient with Kadish stage B disease underwent wide local excision, including endoscopic ethmoidectomy. This individual received post-operative radiotherapy and remains alive, with no evidence of disease at 26 months.
Of the eight patients with Kadish stage C disease, four patients (36 per cent) underwent open anterior craniofacial resection and four patients had a purely endoscopic craniofacial resection. There was a trend for endoscopic resection in the latter years of our timeframe.
One patient received neoadjuvant chemoradiation before open anterior craniofacial resection. Intra-operatively, it was felt that resection would not be curative because of the extent of cranial disease; thus, the right optic nerve and orbit were preserved, leaving a microscopic positive margin. Another patient received neoadjuvant radiotherapy for a 5 cm diameter tumour involving the right orbital apex and frontal lobe, before proceeding to a purely endoscopic craniofacial resection. The in-patient post-operative course was complicated by an acute coronary syndrome. This patient later suffered a fatal myocardial infarction at home four weeks post-operatively, six months following the initiation of therapy. There was no viable residual disease on autopsy.
Of the patients treated endoscopically, one (25 per cent) suffered a CSF leak requiring operative repair. Of the patients treated with open craniofacial resection, one (25 per cent) post-operative course was complicated by two returns to the operating theatre associated with: a haematoma requiring evacuation, and an infected collection requiring washout and removal of bone flap. Despite not proceeding with adjuvant radiation, this patient remains alive without disease after 172 months of follow up. For one other patient in this group, it is unknown whether they proceeded to planned radiation therapy. Post-operative complications occurred in a total of three patients (30 per cent), as described.
A total of six patients were known to have had post-operative radiotherapy, with a median dose of 60 Gy in 30 fractions. Three of these patients also received adjuvant cisplatin chemotherapy. No patients underwent elective treatment of the neck.
The patient with Kadish stage D disease declined surgical intervention, and was treated with chemotherapy and radiotherapy to the primary site and thyroid metastasis. Despite a complete response initially, this patient later re-presented at 113 months with nasal obstruction and local recurrence. The patient declined salvage therapy and remains alive at last follow up (137 months) with symptomatic disease.
Outcomes
Median follow-up duration was 87 months (range, 6–172 months). At last follow up, eight patients (73 per cent) were alive without disease, one patient (9 per cent) had died with no evidence of disease, one patient died with evidence of disease and one patient was alive with evidence of disease.
Three patients (27 per cent) relapsed. The Kadish stage D patient treated with chemoradiotherapy had local recurrence at 113 months. Two patients (18 per cent) had delayed recurrence in the neck nodes occurring outside five years post-primary resection (64 and 78 months, respectively). Both patients had Kadish stage C and Hyams grade 2 disease. Both patients recurred with ipsilateral level II nodal disease; one patient had further ipsilateral level I disease. Neither patient had pathologically positive contralateral nodal disease.
One of the patients with late neck recurrence (at 64 months) received neoadjuvant chemoradiation before undergoing open anterior craniofacial resection, with a microscopic positive resection margin, which was the only such case in our series. This patient recurred with 1 level IIa and 1 level IIb positive node at 64 months. She underwent an ipsilateral modified radical neck dissection (levels I–V, 2 out of 31 nodes were positive) with post-operative radiotherapy. This patient later suffered local recurrence in the frontal bone 109 months after the initial resection (44 months following neck recurrence). She underwent salvage right orbital exenteration and pterional resection with free-flap reconstruction. This procedure was complicated by a CSF leak and positive resection margins. The patient died approximately 1 month later, 110 months following primary resection, of other causes.
The other patient with late neck recurrence (at 78 months) had positron emission tomography CT findings suggestive of right level I–II and left level II nodal disease, and proceeded to bilateral level I–III neck dissection. Pathology revealed 3 out of 28 nodes positive on the right at levels I and II, and 0 out of 32 nodes positive on the contralateral side. Adjuvant radiation to the ipsilateral neck was performed. The patient remains alive, with no evidence of disease, 90 months following primary resection (19 months after neck disease recurrence).
Kaplan–Meier analyses revealed 5-year and 10-year overall survival rates of 90.9 per cent and 68.2 per cent, respectively (Figure 1). Five-year and 10-year disease-free survival rates were 100 per cent and 46.7 per cent, respectively (Figure 2). In a comparison of Kadish stage C patients who underwent open anterior craniofacial resection versus those who underwent purely endoscopic craniofacial resection, there were no significant differences between survival curves for disease-free survival (p = 0.059) or overall survival (p = 0.123).
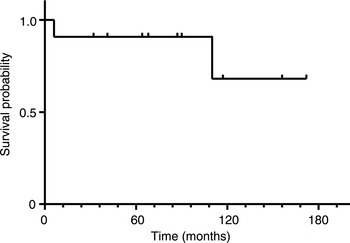
Fig. 1 Overall survival for 11 patients with olfactory neuroblastoma treated with curative intent. Tick marks denote censored observations. Five-year and 10-year overall survival rates were 90.9 per cent and 68.2 per cent, respectively.
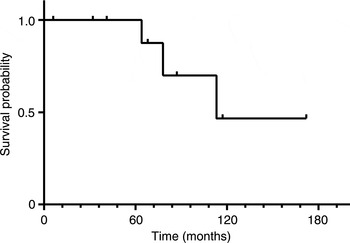
Fig. 2 Disease-free survival for 11 patients with olfactory neuroblastoma treated with curative intent. Tick marks denote censored observations. Five-year and 10-year disease-free survival rates were 100 per cent and 46.7 per cent, respectively.
Discussion
Olfactory neuroblastoma is a rare malignancy of the sinonasal tract. To our knowledge, this is only the second published study of outcomes data from an Australian centre.Reference Nalavenkata, Sacks, Adappa, Palmer, Purkey and Feldman 16 Like other studies, the data are retrospective with low numbers, limiting conclusions. However, given the rarity of this disease, our experience at this tertiary referral centre is valuable in adding to the literature, and in informing future treatment and follow-up protocols.
In published cases, a bimodal age distribution and the absence of gender or race predilection are often noted.Reference Rimmer, Lund, Beale, Wei and Howard 3 In our series, there was an equal distribution of males and females, with the youngest patient presenting at 46 years of age and a median age of 61 years.
Our survival data are comparable to those of previously published series, with five-year disease-free and overall survival rates of 100 per cent and 91 per cent, respectively. A large systematic review of 956 patients from 205 studies reported overall survival rates of 76 per cent and 64 per cent at 5 and 10 years, respectively.Reference Kane, Sughrue, Rutkowski, Aranda, Mills and Buencamino 17 A recent cohort of patients treated at the University of California, Los Angeles, over a similar timeframe to that of our study (2002–2013), had five-year disease-free and overall survival rates of 54 per cent and 82 per cent, respectively.Reference Tajudeen, Arshi, Suh, Palma-Diaz, Bergsneider and Abemayor 18 The most recent study (published in 2016), by Nalavenkata et al., investigated 113 patients across 6 tertiary hospitals in Australia and the USA; they reported a mean overall survival time of 205 months, with a 5-year overall survival rate of approximately 87 per cent.Reference Nalavenkata, Sacks, Adappa, Palmer, Purkey and Feldman 16
The validity of the Kadish classification in risk-stratifying patients is well established, despite inadequacies cited by later authors.Reference Tajudeen, Arshi, Suh, Palma-Diaz, Bergsneider and Abemayor 18 , Reference Bradley, Jones and Robertson 19 Interestingly, the vast majority of our cohort had locally advanced disease, with only one patient with Kadish A and two patients with Kadish B disease. This reflects the tertiary nature of our institution and the delayed presentation often seen with this condition.
Treatment approach remains non-uniform for this disease, although it is widely accepted that multimodality therapy provides the best outcomes. Adequate surgical resection plus adjuvant radiation therapy is the standard of care. In a series of 28 patients, Gruber et al. (in 2002) concluded that radiation alone is insufficient for the treatment of olfactory neuroblastoma, and instead recommended radical resection followed by high-dose post-operative radiation therapy.Reference Gruber, Laedrach, Baumert, Caversaccio, Raveh and Greiner 20 In our cohort, only two patients received surgery alone, one patient had Kadish A disease and the other underwent surgery alone because of post-operative complications. Post-operative radiotherapy status was unknown for one patient. The majority of fit patients received surgery plus adjuvant chemoradiotherapy. Two patients received neoadjuvant therapy to reduce tumour bulk in the setting of Kadish stage C disease with intracerebral extension.
The evolution of endoscopic approaches to this disease has been welcomed given the well-recognised morbidity associated with open craniofacial resection.Reference Ganly, Patel, Singh, Kraus, Bridger and Cantu 21 However, long-term outcome data are lacking, which is important given the propensity of olfactory neuroblastoma to recur many years later. However, recent reviews have demonstrated that in the short to medium term, outcomes are comparable if not superior to traditional open craniofacial resection.Reference Fu, Monteiro, Muhanna, Goldstein and de Almeida 13 At our institution, surgical management has evolved, with purely endoscopic resection being performed from 2006 onwards unless contraindicated. The most recent open anterior craniofacial resection for olfactory neuroblastoma was performed in 2005. All patients undergoing craniofacial resection had Kadish stage C disease. The patient with Kadish stage D disease in our cohort declined surgery and underwent combined chemoradiotherapy with curative intent.
In our cohort, 27 per cent of patients (3 out of 11) experienced disease recurrence outside of five years. The incidence of late recurrence in the neck that occurred in 2 of 11 patients (18 per cent) in our cohort is in keeping with previously reported rates. Petruzzelli et al. reviewed the overall incidence of cervical nodal metastasis across several large series, reporting variation ranging from 5 to 33 per cent.Reference Petruzzelli, Howell, Pederson, Origitano, Byrne and Munoz 2
Beitler et al. first raised the utility of elective neck treatment after a retrospective review of the Memorial Sloan Kettering experience, in which 4 neck failures (29 per cent) were observed in a series of 14 patients treated over a 10-year period.Reference Beitler, Fass, Brenner, Huvos, Harrison and Leibel 22 They also conducted a review of the literature from 1966 to 1991, and found 21 out of 110 cases (19 per cent) of neck failure. In a later study, Demiroz et al. supported the role for elective radiotherapy to the neck in Kadish stage B and C disease, after observing regional failure in 7 of 26 patients (27 per cent) in their series, with the most common site of failure being ipsilateral level II.Reference Demiroz, Gutfeld, Aboziada, Brown, Marentette and Eisbruch 23 The authors also noted the low success of salvage therapy in these patients. A study by Monroe et al. demonstrated a significantly lower rate of neck recurrence in 11 of 20 patients treated with elective neck radiotherapy.Reference Monroe, Hinerman, Amdur, Morris and Mendenhall 24 The overall cervical metastasis rate was 27 per cent, with no neck failures, in the 11 patients who received elective treatment, compared to 4 recurrences in the 9 patients who did not receive elective treatment. However, this finding has not been reported in other series.
In one recent retrospective review of endoscopic surgery cases, two of eight patients (25 per cent) experienced lymph node recurrences, while none had local recurrences.Reference De Bonnecaze, Chaput, Al Hawat, Filleron, Vairel and Serrano 25 A recent multi-institutional series reported neck metastases in 15.9 per cent of patients with olfactory neuroblastoma, with 7.1 per cent primary and 8.8 per cent delayed.Reference Nalavenkata, Sacks, Adappa, Palmer, Purkey and Feldman 16
No patients in our cohort had clinically positive neck disease at presentation or underwent elective neck treatment. Both patients with delayed cervical metastases had advanced local disease at presentation (Kadish C, Hyams grade 2), and underwent endoscopic or endoscopic-assisted resection. One patient had neoadjuvant treatment and also had a positive resection margin. Given the persistent rates of cervical nodal disease and the likelihood of microscopic spread to regional lymph basins in the majority of advanced primary tumours, consideration of prophylactic treatment of the neck must be balanced against the additional morbidity endured by the patient.
-
• Olfactory neuroblastoma is a rare condition with late presentation
-
• Kadish staging and tumour–node–metastasis staging are prognostic
-
• Multimodality therapy is the standard of care
-
• Traditional open anterior craniofacial resection and more recent purely endoscopic resection have comparable outcomes
-
• Delayed cervical nodal failure occurs in up to 30 per cent of patients
-
• Long-term surveillance is recommended; the role for elective neck treatment remains contentious
We support longer-term active surveillance of treated olfactory neuroblastoma, with a particular focus on detecting cervical metastasis early. Patients are generally seen 6-monthly for 5 years, then yearly up to 10 years. This 10-year surveillance programme is designed to identify delayed cervical recurrence, and is targeted particularly at patients who have not had elective treatment to nodal basins. Incorporating radiological surveillance, either with MRI or ultrasound, into the follow-up paradigm is an area for further investigation, and may facilitate early detection and treatment of recurrence. Ultrasound surveillance may be particularly useful in patients from regional and rural centres, allowing follow up closer to home, benefiting the patient, their carers and the health economy. We continue to recommend salvage surgery with post-operative radiotherapy for late cervical nodal failure when it occurs.
Conclusion
Olfactory neuroblastoma is a rare sinonasal malignancy with a variable prognosis. Longer-term follow up, with or without imaging surveillance, is supported given the incidence of late regional and local recurrence observed in this series and in previous literature. The indications for prophylactic treatment of cervical nodes in locally advanced disease, with either radiation or surgical dissection, is an area for further investigation. Prophylactic cervical treatment may also reduce the burden of follow up, particularly for patients from rural and regional areas.




