Introduction
Allergic rhinitis is an inflammatory disorder of the nasal mucosa triggered by immunoglobulin E.Reference Simons1 Although allergic rhinitis does not exhibit a severe course, it compromises one's quality of life and imposes an economic burden on patients.Reference Nalebuff, Cummings, Fredrickson, Harker, Krause and Schuller2 The incidence of allergic rhinitis has increased in recent years, and has been reported to vary between 1.4 per cent and 39.7 per cent.3 The prevalence of allergic rhinitis was reported to have increased by up to 24.1 per cent in China in one cross-sectional study; of these cases, 25.6 per cent had persistent allergic rhinitis.Reference Liu, Zhang, Liu, Zhang, Holtappels and Lin4
There are two forms of allergic rhinitis, intermittent and persistent allergic rhinitis, which are graded in terms of severity as ‘mild’ or ‘moderate to severe’.Reference Bousquet, Khaltaev, Cruz, Denburg, Fokkens and Togias5 Intermittent allergic rhinitis is accompanied by sneezing, watery rhinorrhoea, pruritus, nasal obstruction, vesicle formation and allergic conjunctivitis. In contrast to persistent allergic rhinitis, the symptoms of intermittent allergic rhinitis are not continuous; however, persistent allergic rhinitis is milder.Reference Bousquet, Khaltaev, Cruz, Denburg, Fokkens and Togias5–Reference Lu, Zhao, Zheng, An, Wang and Qiao8 The principal symptom is nasal obstruction.
Eosinophils are the principal effector cells in terms of allergic rhinitis pathogenesis. Cell numbers increase not only in allergic rhinitis patients, but also in those with non-allergic rhinitis with eosinophilia, aspirin hypersensitivity and nasal polyposis.Reference Jalowayski and Zeiger9, Reference Cohen, Ottesen, Middleton, Reed and Ellis10 Exfoliative nasal cytology can be used in the differential diagnosis of allergic rhinitis and non-allergic rhinitis with eosinophilia.Reference Crobach, Hermans, Kaptein, Ridderikhoff and Mulder11
This study aimed to investigate nasal eosinophil levels in persistent allergic rhinitis and intermittent allergic rhinitis, and to compare these data with disease severity and symptom scale scores.
Materials and methods
Patients
The study protocol was approved by the Ethics Committee of Okmeydanı Education Hospital (Istanbul, Turkey). Eighty patients treated in the hospital's out-patient clinic between January 2014 and June 2017 were included. Allergic rhinitis was diagnosed by history-taking, physical examination and the skin prick test. Of the 80 study participants, 60 had allergic rhinitis; these participants were divided into 2 groups. Twenty patients (the control group) were non-symptomatic volunteers with no history of allergic rhinitis. We explored the participants’ eosinophil levels in nasal smears.
The exclusion criteria were: pregnancy, malignant disease, chronic autoimmune disease, acute respiratory tract infection, use of local or systemic anti-inflammatory drugs (e.g. antihistamines, corticosteroids or disodium cromoglicate) in the week prior to diagnostic testing, use of oral corticosteroids in the previous six weeks, use of antihistamines and/or leukotriene receptor antagonists in the preceding two weeks, and a history of polypectomy within the previous six months.
All patients were divided into two groups – intermittent allergic rhinitis and persistent allergic rhinitis – based on the Allergic Rhinitis and its Impact on Asthma system.Reference Bousquet, Khaltaev, Cruz, Denburg, Fokkens and Togias5 Nasal smear status, eosinophil numbers and Total Nasal Symptom Scores were recorded. The eosinophil numbers and Total Nasal Symptom Scores of the persistent and intermittent allergic rhinitis groups were compared.
Nasal smear eosinophilia
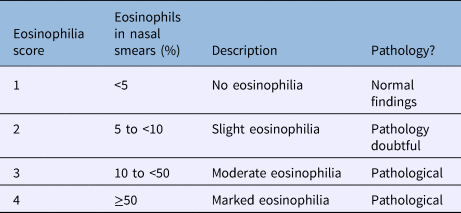
After anterior rhinoscopy, Rhino-Probe™ curettes were used to obtain smears of the inferior meatus mucosae. All cytograms were encoded and read by a single-blinded investigator using light microscopy after haematoxylin and eosin staining. We defined four groups: less than 5 per cent eosinophils (score of 1); 5 to less than 10 per cent eosinophils (score of 2); 10 to less than 50 per cent eosinophils (score of 3), and 50 per cent or more eosinophils (score of 4) (Table 1). A score of 1 was considered to reflect normal findings.
Table 1. Scale used to interpret nasal eosinophilia
Total Nasal Symptom Score
The Total Nasal Symptom Score was used to evaluate nasal symptoms (four in total: nasal obstruction, nasal itching, sneezing and rhinorrhoea). All symptoms were graded between 0 and 3 (0 = absent, 1 = mild, 2 = moderate and 3 = severe).
Statistics
The software IBM SPSS Statistics version 22 (Armonk, New York, USA) was used for all statistical analyses. The Shapiro–Wilk test was employed to evaluate data distributions. Normally distributed data were analysed with the aid of the student's t-test and non-normally distributed data were analysed using the Mann–Whitney U test. The Fisher's exact chi-square test with a continuity correction (that of Yates) was used when analysing non-numerical data. Spearman's rho correlations were employed to compare non-normally distributed data. A p-value of less than 0.05 was considered to reflect significance.
Results
The mean age was 40.03 ± 13.71 years (range, 18–67 years) in the allergic rhinitis patients and 37.10 ± 7.65 years (range, 22–48 years) in the control participants; thus, there was no significant difference. The nasal smear results were pathological in 40 of the 60 allergic rhinitis patients, which was significantly higher than the rate in the control group (p = 0.001). Disease severity did not differ between groups (p > 0.05) (Table 2).
Table 2. Evaluation of allergic rhinitis patients based on disease severity

Data represent numbers (and percentages) of patients, unless indicated otherwise. Analysed using Yates’ correction for continuity.
There was no difference between the persistent and intermittent allergic rhinitis groups in terms of Total Nasal Symptom Scores (p > 0.05) (Table 3). However, the mean nasal eosinophilia score of the intermittent allergic rhinitis group was significantly higher than that of the persistent allergic rhinitis group (p = 0.029) (Table 3).
Table 3. Nasal eosinophilia scores and Total Nasal Symptom Scores of allergic rhinitis patients

Data represent mean ± standard deviation (median) score, unless indicated otherwise. Analysed using the Mann–Whitney U test. *p < 0.05.
We found no significant difference in the nasal eosinophilia score distribution. A score of 1 was most common in the persistent allergic rhinitis group and a score of 3 in the intermittent allergic rhinitis group (p > 0.05) (Table 4). A significant difference in score distribution between those with mild and moderate to severe disease was evident (p < 0.05) (Table 5).
Table 4. Evaluation of nasal eosinophilia scores

Data represent numbers (and percentages) of patients, unless indicated otherwise. Analysed using the chi-square test.
Table 5. Nasal eosinophilia level by disease severity

Data represent numbers (and percentages) of patients, unless indicated otherwise. Analysed using the chi-square test. *p < 0.05.
A positive correlation was evident between the nasal eosinophilia score and Total Nasal Symptom Score (r = 0.652; p < 0.05) in allergic rhinitis patients (Table 6).
Table 6. Correlations between nasal eosinophilia scores and Total Nasal Symptom Scores of allergic rhinitis patients

Analysed using the Spearman's rho test. *p < 0.05.
Discussion
The primary aim of our study was to compare eosinophilia and symptoms in persistent allergic rhinitis and intermittent allergic rhinitis patients. We found that both persistent and intermittent allergic rhinitis patients exhibited increased eosinophilia levels, and that persistent allergic rhinitis caused a greater increase. Eosinophils act as effector cells during the pathogenesis of allergic inflammation, and their numbers are increased in the blood and nasal secretions of atopic subjects.Reference Skoner12 Eosinophils and mast cells play key roles in immunoglobulin E mediated allergic reactions, including allergic rhinitis.Reference Boyce, Broide, Matsumoto and Bochner13, Reference Prussin and Metcalfe14 Although eosinophil levels may double, smear findings can be normal during asymptomatic periods.Reference Simons1 One problem is that no consensus method of smear evaluation has yet emerged. Thus, the published results vary widely.Reference Crobach, Hermans, Kaptein, Ridderikhoff and Mulder11, Reference Sood15–Reference Jankowski, Persoons, Foliguet, Coffinet, Thomas and Verient-Montaut18 Lans et al.Reference Lans, Alfano and Rocklin16 and Jankowski et al.Reference Jankowski, Persoons, Foliguet, Coffinet, Thomas and Verient-Montaut18 used 20 per cent as a cut-off value, whereas Burrows et al.Reference Burrows, Hasan, Barbee, Halonen and Lebowitz17 employed 25 per cent and SoodReference Sood15 adopted 5 per cent, as did Crobach et al.Reference Crobach, Hermans, Kaptein, Ridderikhoff and Mulder11
Although the nasal smear has been proposed as a useful tool for the diagnosis of allergic rhinitis, no consensus exists.Reference Gelardi, Fiorella, Russo, Fiorella and Ciprandi19, Reference Ahmadiafshar, Taghiloo, Esmailzadeh and Falakaflaki20 The nasal smear test is inexpensive, rapid, non-invasive and simple, yielding information on both acute and chronic rhinosinusitis.Reference Lans, Alfano and Rocklin16, Reference Gelardi, Fiorella, Russo, Fiorella and Ciprandi19, Reference Nurkic, Ahmad, Arifhodzic and Jusufovic21, Reference Bryan and Bryan22 Bousquet et al.Reference Bousquet, Khaltaev, Cruz, Denburg, Fokkens and Togias5 concluded that raised eosinophilia alone was not adequate for the diagnosis of allergic rhinitis. On the contrary, Bryan and BryanReference Bryan and Bryan22 found that the mucosae of allergic rhinitis patients exhibited increased eosinophilia while normal mucosae did not. Malmberg et al.Reference Malmberg and Holopainen23 found a correlation between eosinophilia and allergic rhinitis. SoodReference Sood15 reported nasal eosinophilia in 80 per cent of allergic rhinitis patients and in only 5 per cent of controls, but emphasised that eosinophilia in the control group was very mild. VaheriReference Vaheri24 found an 80 per cent and Bhandari and BaldwaReference Bhandari and Baldwa25 an 81.6 per cent rate of eosinophilia in allergic rhinitis patients. We found a slightly lower rate.
In general, eosinophil numbers tend to be lower in those with constant, compared with seasonal, disease, suggesting that the extent of eosinophilia depends on the extent of seasonal allergen exposure. Eosinophilia and mast cell numbers were higher in intermittent and persistent allergic rhinitis groups compared with controls, and persistent allergic rhinitis patients had higher cell numbers than intermittent allergic rhinitis patients in a Chinese population.Reference Liu, Zhang, Liu, Zhang, Holtappels and Lin4 In contrast, we found a lower rate of eosinophilia in persistent allergic rhinitis patients. Of all smears, 56.4 per cent were positive in the persistent allergic rhinitis group and 85.7 per cent were positive in the intermittent allergic rhinitis group. Control patients had normal smear results, but, during exacerbation periods, the intermittent allergic rhinitis eosinophilia scores were 3 and 4 in 52.4 per cent and 19 per cent of patients, respectively.
• Allergic rhinitis incidence has increased, varying between 1.4 and 39.7 per cent
• Allergic rhinitis is intermittent or persistent, and graded as ‘mild’ or ‘moderate to severe’
• The nasal smear results were pathological in 40 of 60 allergic rhinitis patients, significantly higher than the rate in controls
• Nasal eosinophilia score and Total Nasal Symptom Score were positively correlated (r = 0.652; p < 0.05) in persistent and intermittent allergic rhinitis patients
• The nasal smear test is inexpensive, objective and simple, and should be a part of the diagnostic investigation
• Skin prick test positivity is related to allergic rhinitis; results can be used diagnostically and for treatment planning
Okano et al.Reference Okano, Nishizaki, Nakada, Kawarai, Goto and Satoskar26 stated that nasal eosinophilia could be a predictor of symptoms, and that both eosinophilia per se and symptoms could be used to guide treatment. SoodReference Sood15 considered that nasal eosinophilia was a highly sensitive and specific marker of allergic rhinitis. Lans et al.Reference Lans, Alfano and Rocklin16 found nasal eosinophilia in 43 per cent of allergy patients but not in any skin prick test negative subjects. They considered that smear positivity lacked sensitivity but was specific for allergic rhinitis. Perić et al.Reference Perić, Vojvodić and Vukomanović-Durdevid27 reported that eosinophil count was higher in cases of nasal polyposis than in non-atopic patients. We found positive correlations between Total Nasal Symptom Scores and both persistent and intermittent allergic rhinitis positive patients (both p < 0.05).
Conclusion
Nasal eosinophil levels were elevated in both intermittent and persistent allergic rhinitis patients. The extent of eosinophilia and the Total Nasal Symptom Score correlated well. The nasal smear is an inexpensive, objective and simple test, and should be part of the diagnostic investigation.
Competing interests
None declared.








