Introduction
The nose is a unique organ in that it exhibits asymmetrical airflow, with the dominant airflow alternating from one nasal passage to the other over a period of hours.Reference Eccles 1 This alternation of nasal airflow is often described as a ‘nasal cycle’ as it can be regular and reciprocal. For many centuries, the ancient art of yoga has studied nasal breathing and developed techniques to switch the nasal airflow from one side to the other by the use of a small crutch applied to the axilla (a yoga danda).Reference Bhole and Karambelkar 2 The yoga belief is that nasal airflow influences brain activity depending on whether airflow is dominant through the right or left nasal passage; hence, by controlling nasal airflow with the yoga danda, the yoga student can control brain activity.Reference Shannahoff-Khalsa 3
The effects of the yoga danda on nasal airflow have been confirmed in several studies in different research centres, and this ancient practice now has scientific support: reciprocal changes in nasal airflow can be caused by pressure applied to the axilla by means of a small crutch, or by adopting the lateral recumbent position.Reference Rao and Potdar 4 – Reference Haight and Cole 6 However, whether this extends to an influence on brain activity remains controversial.
This review examines the evidence that links nasal airflow and brain activity in relation to two current ideas: firstly, the proposal that asymmetrical brain activity causes asymmetrical nasal airflow, and, secondly, that asymmetrical nasal airflow causes asymmetrical brain activity.
Materials and methods
A Medline search was conducted using the following key words: nasal airflow, nasal cycle, nasal hyperventilation, forced nostril breathing, brain asymmetry, electroencephalogram (EEG), cerebral activity, cognition, cerebral lateralisation, epilepsy, autism and schizophrenia. Reference lists were hand-searched for other articles of interest.
Results
Does asymmetrical brain activity cause asymmetrical nasal airflow?
Nasal airflow control
Changes in nasal airflow are mediated by alternating dilation and constriction of veins in the nasal mucosa, by action of the sympathetic nervous system.Reference Hanif, Jawad and Eccles 7 Studies on anaesthetised cats have suggested that the central control of sympathetic tone involved collections of sympathetic neurons in the brainstem, so-called ‘oscillators’.Reference Bamford and Eccles 8 The dominance of sympathetic output was found to alternate from the left to right oscillator and vice versa, resulting in reciprocal changes to nasal airflow.Reference Bamford and Eccles 8
Animal studies have suggested that overall control occurs at the level of the hypothalamus, as electrical stimulation here causes an overall increase in sympathetic tone and greater nasal airflow bilaterally.Reference Eccles and Lee 9 Therefore, with the hypothalamus as the generator of a rhythmic nasal cycle, increased or decreased hypothalamic output will stimulate the brainstem oscillators symmetrically, but these oscillators will influence nasal airflow asymmetrically due to their reciprocal differences in sympathetic discharge.Reference Williams and Eccles 10 Cortical influence on the hypothalamus and brainstem in terms of the nasal cycle is yet to be established; however, there is some evidence to support a link between cortical functions and nasal airflow, as discussed below.
Fixed cerebral asymmetries and nasal airflow
The cerebral hemispheres exhibit both functional and structural asymmetry;Reference Sun and Walsh 11 for example, hand preference. Searleman and colleagues, in 2005, hypothesised a correlation between nasal airflow and handedness, based on the observation that there is often a consistency in lateral preferences (e.g. left-handers tend to be left-footed, left-eyed and so on).Reference Searleman, Hornung, Stein and Brzuszkiewicz 12 They found that the dominant nostril positively correlated with the dominant hand for almost 60 per cent of the time.Reference Searleman, Hornung, Stein and Brzuszkiewicz 12 However, the study only involved a small cohort monitored over a short time period, and, as previously demonstrated, there is great variability in patterns of nasal airflow.Reference Flanagan and Eccles 13 It strikes us as unusual that this phenomenon has not been noted in other observational studies of healthy individuals. Given that 90 per cent of the population are right-handed, it would seem likely that another study would have documented the finding of a dominant right nostril, even just incidentally.
Left or inconsistent handedness seems to be more prevalent than expected in certain neurodevelopmental and psychiatric disorders such as autism and schizophrenia, which may be related to cerebral lateralisation abnormalities.Reference Dane, Yildirim, Ozan, Aydin, Oral and Ustaoglu 14 – Reference Satz and Green 16 One study analysed hand preference and nasal airflow in autistic children, and found that the majority were left-handed and had left nostril dominance for most of the time.Reference Dane and Balci 15 Another study in right-handed schizophrenics revealed a significant increase in left nostril dominance in this group compared with controls.Reference Dane, Yildirim, Ozan, Aydin, Oral and Ustaoglu 14
Handedness is in fact a continuum, with degrees of left- and right-handedness.Reference Beaton, Hugdahl and Davidson 17 Furthermore, different methods of measurementReference Oldfield 18 , Reference Brown, Roy, Rohr, Snider and Bryden 19 are not standardised across studies, making interpretation difficult. It should also be noted that the above studies contain small sample sizes, with possible confounding factors that were not controlled for, such as the use of psychoactive medication.
Fluctuating cerebral asymmetries and nasal airflow
The idea of rhythmic, spontaneous fluctuations in cerebral hemisphere activity first appeared in the 1960s. Following the discovery of the rapid/non-rapid eye movement sleep cycle,Reference Aserinsky and Kleitman 20 Kleitman, in 1967, proposed that this phenomenon was the nocturnal part of a ‘basic rest–activity cycle’, which involves fluctuations in brain activity approximately every 90 minutes (an ultradian rhythm).Reference Kleitman 21 However, the exact nature of these changes in brain activity remains contentious, and conflicting results have been presented.Reference Klein, Pilon, Prosser and Shannahoff-Khalsa 22 , Reference Neubauer and Freudenthaler 23
Based on the basic rest–activity cycle theory, a correlation between the alternating pattern of nasal airflow and the alternating fluctuations in brain activity has been suggested.Reference Klein, Pilon, Prosser and Shannahoff-Khalsa 22 , Reference Werntz, Bickford, Bloom and Shannahoff-Khalsa 24 Werntz et al., in 1983, discovered relatively greater EEG activity in the hemisphere contralateral to the dominant nostril, as measured by nasal airflow in 19 subjects.Reference Werntz, Bickford, Bloom and Shannahoff-Khalsa 24 A larger study involving 126 right-handed participants found a tendency for enhanced performance in verbal tasks at times of right nostril dominance, and enhanced performance in spatial tasks at times of left nostril dominance (i.e. a link between nostril dominance and the contralateral hemisphere).Reference Klein, Pilon, Prosser and Shannahoff-Khalsa 22 However, EEG studies in particular are difficult to interpret, and have varying methods of analysis with a high level of inter-individual variability.Reference Manseau and Broughton 25
Model of how brain influences nasal airflow
The model illustrated in Figure 1 summarises the evidence and ideas presented in this section. For simplicity, the control is discussed from the peripheral nerves, moving upwards through the hierarchy of central nervous control centres. The peripheral control of nasal airflow via the autonomic nervous system is well documented,Reference Hanif, Jawad and Eccles 7 , Reference Stoksted and Thomsen 26 and involves the vasoconstrictor sympathetic nerves that supply the large veins in the turbinates. The asymmetry in brain activity and sympathetic tone extend to the brainstem region, where left and right oscillators cause reciprocal changes in nasal airflow.Reference Bamford and Eccles 8 The hypothalamus may provide the overall rhythmicity to a cycle of reciprocal changes in nasal airflow, but there is no evidence for asymmetry at this level.Reference Eccles and Lee 9 Cortical involvement in nasal airflow asymmetry has been suggested by studies on handedness,Reference Searleman, Hornung, Stein and Brzuszkiewicz 12 ultradian rhythms of cerebral activity,Reference Werntz, Bickford, Bloom and Shannahoff-Khalsa 24 and lateralisation disorders such as schizophreniaReference Dane, Yildirim, Ozan, Aydin, Oral and Ustaoglu 14 and autism,Reference Dane and Balci 15 but the evidence for these influences is weak and this area of research is controversial.
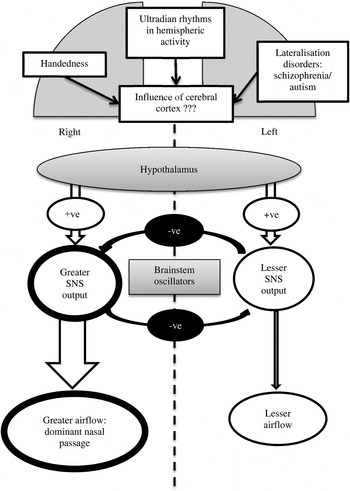
Fig. 1 Model to explain the influence of brain activity on nasal airflow. The overall alternation in sympathetic nervous system (‘SNS’) output over a period of hours is controlled from the hypothalamus, and the asymmetry in sympathetic outflow is determined by the activity of brainstem oscillators which act as a flip-flop mechanism, with each centre inhibiting the activity of the other centre and only one centre having dominant activity at any one time.Reference Bamford and Eccles 8 , Reference Eccles and Lee 9 Higher centres in the cerebral cortex may also influence nasal airflow, leading to asymmetry.Reference Searleman, Hornung, Stein and Brzuszkiewicz 12 , Reference Dane and Balci 15 +ve = positive; −ve = negative
Does asymmetrical nasal airflow cause asymmetrical brain activity?
The sensation of nasal airflow is provided by stimulation of nasal trigeminal nerve endings that detect cooling of the nasal mucosa, as occurs during inspiration.Reference Eccles 1 , Reference Sozansky and Houser 27 A purely trigeminal stimulus has been shown to increase arousal frequency and duration during sleep,Reference Heiser, Baja, Lenz, Sommer, Hormann and Herr 28 whereas no effect was seen with an olfactory stimulus.Reference Heiser, Baja, Lenz, Sommer, Hormann and Herr 29 Therefore, it seems that a nasal airflow stimulus can influence brain activity. Further evidence is discussed below.
Nasal hyperventilation and epileptic activity
Studies have demonstrated that deep breathing can activate epileptic foci, triggering seizure activity.Reference Servit, Kristof and Strejckova 30 Although this was previously explained by hypocarbia leading to vasoconstriction and cerebral ischaemia, it may actually be related to airflow stimulating the nasal mucosa.Reference Servit, Kristof and Strejckova 30 Insufflation of air into the nasal cavity has been shown to trigger epileptic areas in the brain in animal studies.Reference Servit, Kristof and Strejckova 30 In certain types of human epilepsy, nasal hyperventilation was more likely than oral hyperventilation to stimulate epileptic EEG activity, and unilateral nostril breathing had a greater effect on abnormalities in the ipsilateral hemisphere.Reference Servit, Kristof and Strejckova 30 , Reference Servit, Kristof and Kolinova 31 This effect was suppressed following application of local anaesthetic to the nasal mucosa around the superior meatus.Reference Servit, Kristof and Strejckova 30 , Reference Kristof, Servit and Manas 32 The exact mechanism of this phenomenon is not fully established; however, the authors of the above studies have suggested a reflex involving stimulation of the olfactory nerve by nasal airflow.Reference Servit, Kristof and Strejckova 30 , Reference Kristof, Servit and Manas 32
Unilateral forced nostril breathing
Asymmetrical nasal airflow with unilateral forced nostril breathing, where one nostril is occluded either manually by the subject or with cotton wool, has been used to analyse the influence of asymmetrical nasal airflow on the brain, as measured by EEG activityReference Williams and Eccles 10 and cognitive performance.Reference Sun and Walsh 11 , Reference Joshi and Telles 33 In fact, these concepts have their basis in ancient yogic practices, and there is a relatively large body of literature discussing nasal breathing methods utilised in yoga and their effects on mood and cognition.Reference Joshi and Telles 33 – Reference Desai, Tailor and Bhatt 37
Several studies have demonstrated that unilateral forced nostril breathing affects the autonomic nervous system, by changing cardiovascular parameters for example.Reference Dane, Caliskan, Karasen and Oztasan 38 – Reference Shannahoff-Khalsa and Kennedy 40 In a study of five subjects, unilateral forced nostril breathing caused a shift in the dominant hemisphere, as measured by relatively greater EEG activity, often within 2 minutes.Reference Werntz, Bickford and Shannahoff-Khalsa 41 Other studies have used hemisphere-specific tasks to measure cognitive performance, as verbal tasks reflect left hemisphere activity and spatial tasks reflect right hemisphere activity. The results have been conflicting. One study identified significant improvements in verbal test scores with right unilateral forced nostril breathing and improvements in spatial test scores with left unilateral forced nostril breathing.Reference Shannahoff-Khalsa, Boyle and Buebel 42 Others found that left unilateral forced nostril breathing significantly improved right hemisphere performance, whereas right unilateral forced nostril breathing had no effect.Reference Joshi and Telles 33 , Reference Jella and Shannahoff-Khalsa 43 The opposite effect has also been reported, wherein right unilateral forced nostril breathing improved left hemisphere performance but left unilateral forced nostril breathing had no effect.Reference Telles, Joshi and Somvanshi 36 Several studies have failed to show that unilateral forced nostril breathing has any effect on EEG measurementsReference Velikonja, Weiss and Corning 44 or cognitive performance.Reference Klein, Pilon, Prosser and Shannahoff-Khalsa 22 Unilateral forced nostril breathing has also been reported to affect emotional responses.Reference Schiff and Rump 45
Often these studies are difficult to interpret accurately and have conflicting results. Problems include small sample sizes,Reference Werntz, Bickford and Shannahoff-Khalsa 41 , Reference Shannahoff-Khalsa, Boyle and Buebel 42 , Reference Block, Arnott, Quigley and Lynch 46 differing methods of measuring cognitive performanceReference Shannahoff-Khalsa, Boyle and Buebel 42 , Reference Block, Arnott, Quigley and Lynch 46 and failure to consider potential confounding factors such as handedness.Reference Werntz, Bickford and Shannahoff-Khalsa 41
From observations of an overall left nostril dominance in autistic children, it has been hypothesised that the enhanced visuospatial abilities and lack of speech development often seen in this group could be due to continuous stimulation of the right hemisphere by predominantly left nasal airflow.Reference Dane and Balci 15 It would seem that if this were true, a correlation between unilateral nasal blockage (e.g. septal deviation) and autism would have been established by now. Shannahoff-Khalsa and his colleagues have suggested in multiple articles that unilateral forced nostril breathing has potential as a non-invasive treatment for psychiatric disorders,Reference Werntz, Bickford, Bloom and Shannahoff-Khalsa 24 , Reference Werntz, Bickford and Shannahoff-Khalsa 41 , Reference Shannahoff-Khalsa 47 and recorded a correlation between left nostril dominance and hallucination occurrence in one schizophrenic female.Reference Shannahoff-Khalsa and Golshan 48 Unilateral forced nostril breathing may also have beneficial effects for speech recovery in stroke patients.Reference Marshall, Laures-Gore, DuBay, Williams and Bryant 49
Model of how nasal airflow influences brain activity
The model illustrated in Figure 2 summarises the evidence and ideas presented in this section. Inspired air stimulates cold receptors in the nasal mucosa innervated by the trigeminal nerve, providing the sensation of nasal airflow. Environmental sensory stimuli such as noise or smells can enhance arousal, and this effect is mediated by the reticular formation – an area in the brainstem involved in arousal and consciousness.Reference Moruzzi and Magoun 50 Insufflation of air into the nose has been shown to cause increased arousal, as demonstrated by EEG changes in animal studies.Reference Arduini and Moruzzi 51 Therefore, a nasal airflow stimulus, such as insufflation of air or unilateral forced nostril breathing, could activate the reticular formation and increase arousal, leading to EEG changes and possibly improved cognitive performance. There is evidence to suggest both ipsilateralReference Servit, Kristof and Strejckova 30 , Reference Block, Arnott, Quigley and Lynch 46 and contralateralReference Joshi and Telles 33 , Reference Werntz, Bickford and Shannahoff-Khalsa 41 , Reference Shannahoff-Khalsa, Boyle and Buebel 42 stimulating effects. However, we propose it is more likely that a unilateral nasal airflow stimulus has an activating effect on both cerebral hemispheres, but with a greater effect on one side. As trigeminal neurons transmitting temperature signals cross the midline in the medulla, it seems logical that the greatest effect would be seen contralaterally.

Fig. 2 Model to explain the influence of nasal airflow on brain activity. A nasal airflow stimulus such as unilateral forced nostril breathing stimulates trigeminal nerve endings on one side of the nose.Reference Sozansky and Houser 27 Trigeminal neurons transmitting temperature signals synapse in the spinal trigeminal nucleus and then cross the midline, travelling up to the thalamus through the brainstem. Via the brainstem reticular formation, a nasal airflow stimulus could lead to enhanced arousal and brain activity in both cerebral cortices.Reference Moruzzi and Magoun 50 , Reference Arduini and Moruzzi 51 Studies have intimated that the greatest stimulating effect occurs in the hemisphere contralateral to the nasal airflow stimulus.Reference Joshi and Telles 33 , Reference Telles, Joshi and Somvanshi 36 , Reference Werntz, Bickford and Shannahoff-Khalsa 41 , Reference Shannahoff-Khalsa, Boyle and Buebel 42
Discussion
The presence of asymmetrical nasal airflow that fluctuates spontaneously throughout the day has been established in multiple studies.Reference Hasegawa and Kern 52 – Reference Gilbert and Rosenwasser 54 The role of higher centres and cortical organisation in the control of nasal airflow remains uncertain, with some conflicting theories suggested. For example, if nasal airflow dominance correlates with hand preference,Reference Searleman, Hornung, Stein and Brzuszkiewicz 12 it could not also correlate with fluctuating ultradian rhythms of cerebral activity,Reference Klein, Pilon, Prosser and Shannahoff-Khalsa 22 as hand preference is fixed.
Based on ancient yoga breathing techniques, evidence is emerging which suggests that altering nasal airflow can influence brain activity.Reference Werntz, Bickford and Shannahoff-Khalsa 41 , Reference Jella and Shannahoff-Khalsa 43 , Reference Block, Arnott, Quigley and Lynch 46 Considering the evidence from studies of epileptic patientsReference Servit, Kristof and Strejckova 30 , Reference Kristof, Servit and Manas 32 and arousal during sleep,Reference Heiser, Baja, Lenz, Sommer, Hormann and Herr 28 it seems that a nasal airflow stimulus has some sort of activating effect on the brain. Putting this into an everyday context, stepping outside and inhaling cool air through the nose often makes us feel more alert, and the cooling properties of menthol on nasal receptors have a similar effect.Reference Eccles 55 Smelling salts were used in Victorian times to revive unconscious patients, and even nowadays some athletes use smelling salts as a stimulant prior to competing.Reference McCrory 56
A proposed mechanism for a correlation between nasal airflow and cerebral hemisphere activity involves the sympathetic nervous system,Reference Werntz, Bickford, Bloom and Shannahoff-Khalsa 24 , Reference Werntz, Bickford and Shannahoff-Khalsa 41 , Reference Jella and Shannahoff-Khalsa 43 supported in part by the other autonomic effects found to occur during unilateral forced nostril breathing.Reference Dane, Caliskan, Karasen and Oztasan 38 – Reference Shannahoff-Khalsa and Kennedy 40 As autonomic nerve fibres connecting the nose and hypothalamus do not decussate, vasoconstriction in the nasal vessels has been postulated to reflect concurrent vasoconstriction in the ipsilateral cerebral hemisphere, leading to a decrease in cerebral blood flow ipsilaterally and a relative increase contralaterally.Reference Werntz, Bickford, Bloom and Shannahoff-Khalsa 24 , Reference Werntz, Bickford and Shannahoff-Khalsa 41 , Reference Jella and Shannahoff-Khalsa 43 In this way, the increased blood flow could improve cognitive function as measured by performance in hemisphere-specific tasks. However, we question the physiological basis for this theory. Task performance has been shown to increase overall blood flow to both hemispheres; more specifically, verbal tasks cause a left lateralisation of blood flow and spatial tasks a right lateralisation in right-handed subjects.Reference Vingerhoets and Stroobant 57 , Reference Schmidt, Krings, Willmes, Roessler, Reul and Thron 58 The effect of the sympathetic nervous system on cerebral blood flow in the absence of pathology is thought to be minimal due to the action of cerebral autoregulation.Reference ter Laan, van Dijk, Elting, Staal and Absalom 59 In fact, whilst blockade of the stellate ganglion (i.e. inhibition of sympathetic activity) increases blood flow in extracranial vessels, it has no effect intracranially.Reference Kang, Oh, Chung, Lee, Park and Kim 60
Therefore, we have proposed a different mechanism for the effect of nasal airflow on brain activity, incorporating the activating effect of a nasal airflow stimulus on the cerebral cortex via the reticular formation, as illustrated in Figure 2. One major challenge is that the laterality of cerebral hemisphere stimulation by nasal airflow is unclear, with some studies suggesting an ipsilateral responseReference Servit, Kristof and Kolinova 31 , Reference Block, Arnott, Quigley and Lynch 46 and others a contralateral response.Reference Werntz, Bickford and Shannahoff-Khalsa 41 – Reference Jella and Shannahoff-Khalsa 43 Olfactory nerve fibres do not decussate and therefore principally stimulate the ipsilateral cortex, whereas trigeminal fibres relaying temperature signals cross the midline before passing through the brainstem. The trigeminal nerve detects nasal airflow, but experimental insufflation of air could stimulate the olfactory nerve due to the inadvertent presence of an olfactory stimulus. In addition, the olfactory cortex is unable to sense the laterality of a stimulus unless the trigeminal nerve is also stimulated.Reference Kobal, Van Toller and Hummel 61 Suppression of the EEG stimulation caused by nasal airflow associated with application of local anaesthetic to the nasal mucosaReference Kristof, Servit and Manas 32 is more suggestive of trigeminal nerve involvement. Although sleep studies have demonstrated arousal secondary to a trigeminal nerve stimulus, this stimulus was an irritant (carbon dioxide)Reference Heiser, Baja, Lenz, Sommer, Hormann and Herr 28 and is therefore difficult to compare with nasal inspiration of air as in unilateral forced nostril breathing.
It is possible that nasal airflow causes bilateral cortical stimulation, with a greater effect on one side. Experimental stimulation of the reticular formation in anaesthetised cats caused EEG changes indicating increased alertness, and at lower levels of stimulation this effect was only seen in the ipsilateral hemisphere.Reference Moruzzi and Magoun 50 It is unclear whether the trigeminal or olfactory nerves are involved in this mechanism.
Conclusion
The ancient yogic practice of breath control exercises are thought to promote health and well-being, improve circulation, and prepare one for concentration.Reference Iyengar 62 Whilst this notion may have been met with scepticism from the scientific community, it has inspired clinical studies into the effects of nasal breathing on cognition. There is a growing body of evidence to suggest that nasal airflow can influence brain activity; however, the mechanism, extent and significance are debatable.




