Introduction
The term ‘myopericytoma’ was coined in 1998 to describe a benign neoplasm with a striking perivascular proliferation of spindle cells and differentiation towards perivascular myoid cells or pericytes.Reference Granter, Badizadegan and Fletcher1 Myopericytoma is closely related to the spectrum of tumours that demonstrate pericytic differentiation, including myofibromatosis, solitary myofibroma and infantile haemangiopericytoma. It most commonly develops in middle adulthood.Reference Cao, Xu, Li, Lai and Li2 Myopericytomas are typified by a predilection for skin and soft tissue, and involvement of the extremities.Reference Woollard, Southgate and Blair3 However, the tumour is receiving increasing clinicopathological recognition as a distinct entity, which has resulted in an expanding, evolving anatomical distribution.Reference Cao, Xu, Li, Lai and Li2 Myopericytomas have very occasionally been documented in the head and neck area.Reference Cao, Xu, Li, Lai and Li2, Reference Datta, Rawal, Mincer and Anderson4–Reference Wilson, Hellquist, Ray and Pickles7 However, an extensive literature review using the PubMed search engine confirmed that aural involvement remains unreported in the English-language literature.
In documenting a polypoid myopericytoma of the external auditory canal and tragus, we aimed to heighten awareness of this tumour in the head and neck area. We propose that myopericytoma is added to the list of tumours that present with polypoid architecture in this location, and discuss the potential diagnostic pitfalls and mimicry.
Case report
Clinical features
An 18-year-old (human immunodeficiency virus negative) woman sought medical attention, for cosmetic reasons, for a slow-growing mass that involved the left external auditory canal and tragus, which had developed over the previous 9 months (Figure 1a). The mass was not associated with pain, rapid growth, bleeding, or impedance to hearing or jaw movement. There were no other otorhinolaryngological complaints. The patient's past medical, surgical and family history was non-contributory.
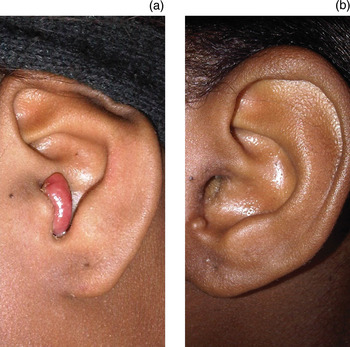
Fig. 1 (a) Pre-operative view of the patient showing a polypoid mass involving the external auditory canal and tragus, and (b) the post-operative view at six months follow up.
Examination revealed an otherwise healthy woman with a 20 × 20 mm, firm, smooth, well demarcated, non-tender, polypoid mass involving the lateral aspect of the left tragus, with no overlying skin changes. The findings of the otoscopic examination of the external auditory canal and tympanic membrane were normal. The Rinne tuning fork test (a clinical hearing test) was positive for frequencies 256, 512 and 1023 Hz bilaterally. There was no cervical lymphadenopathy or abnormalities of the skin and mucous membranes.
Incisional biopsy of the mass confirmed a spindle cell tumour with a myoid immunophenotype. Excision biopsy of the mass was conducted under general anaesthetic. The excision biopsy findings revealed incomplete excision margins. Wide local excision of the tumour site and left tragus was subsequently performed and tumour-free margins were confirmed.
The patient was well at follow up, with no recurrence at one, three or six months post-excision (Figure 1b).
Pathological features
On gross examination, the excised tumour measured 12 × 10 × 7 mm in size. It had a firm consistency and a grey-white appearance on cut section.
On microscopic assessment, the tumour demonstrated the presence of blood vessels of varying thickness, with a perivascular concentric arrangement of ovoid to plump spindle-shaped cells (Figure 2). In some fields, the spindle-shaped cells appeared to spin off the vessel walls. Thin-walled vessels, some with an antler-like, haemangiopericytomatous architecture, were also evident (Figure 2). The mitotic activity was less than 1 mitotic figure per 10 high-power fields. Cellular pleomorphism, tumoural necrosis and lymphovascular invasion were not seen. Admixed lymphocytes and plasma cells were noted. The tumour cells demonstrated muscle-specific actin (Figure 3a), alpha smooth muscle actin (Figure 3b), h-caldesmon immunopositivity (Figure 4a), desmin immunonegativity (Figure 4b) and Epstein–Barr virus-encoded RNA negativity. A diagnosis of myopericytoma was made.

Fig. 2 Microscopic view of the vascularised spindle cell tumour, with staghorn-type vessel architecture (asterisk) and vessels surrounded by whirls of plump myoid cells (arrows). (H&E; ×240)

Fig. 3 Microscopic view of the tumour cells showing the immunohistochemistry staining for (a) muscle-specific actin and (b) alpha smooth muscle actin. (×480)

Fig. 4 Microscopic view of the tumour cells with immunohistochemistry staining showing (a) h-caldesmon immunopositivity (arrow) and (b) desmin immunonegativity. (×480)
Discussion
The pericyte, first described by Zimmermann in 1923, is a pluripotent cell that may differentiate into smooth muscle cells, adipocytes and osteoblasts.Reference Mentzel, Dei Tos, Sapi and Kutzner8 In coining the term ‘myopericyte’ in 1992, Dictor et al. referred to it as a cell transitional between pericytes and smooth muscle cells of vessels.Reference Paek, Kang, Yeo, Yu and Kim9 Neoplasms that display putative pericytic differentiation were first documented as haemangiopericytomas by Stout and Murray in 1942; however,Reference Mentzel, Dei Tos, Sapi and Kutzner8 the lack of reliable and reproducible diagnostic features for haemangiopericytoma has resulted in an ill-defined entity that is now largely regarded as a growth pattern rather than a distinct tumour.Reference Fletcher10 It is only in the last 13 years that myopericytoma has been increasingly recognised and defined as a distinct entity.Reference Granter, Badizadegan and Fletcher1–Reference Paek, Kang, Yeo, Yu and Kim9, Reference Dray, McCarthy, Palmer, Bonar, Stalley and Marjoniemi11–Reference Xia, Chen, Geng, Jiang, Yang and Zhang16 Myopericytoma is categorised as a tumour that originates from the perivascular myoid cell, and it shares features of smooth muscle and glomus cells.Reference Dray, McCarthy, Palmer, Bonar, Stalley and Marjoniemi11 Myopericytoma is currently recognised by the World Health Organization as belonging to a subset of perivascular tumours with myoid features.Reference McMenamin, Fletcher, Unni and Mertens13
Most reported myopericytomas are slow-growing, benign tumours with a good prognosis. However, local recurrences have been recorded; these are most commonly the result of incomplete excision.Reference Wilson, Hellquist, Ray and Pickles7 The management of myopericytomas involves local excision, with further excision if there is recurrence.
Since its first description, the clinicopathological spectrum has expanded to include tumours occurring in intravascular locations,Reference McMenamin and Calonje14 those associated with the Epstein–Barr virus infection and immunosuppression,Reference Lau, Wong, Lui, Cheung, Ho and Wong5 and tumours with a malignant profile.Reference Mentzel, Dei Tos, Sapi and Kutzner8 Malignant myopericytoma, which is rarely reported, is typified by disseminated disease and patient mortality.Reference Ramdial, Sing, Deonarain, Singh, Allopi and Moodley15
To date, myopericytoma has most commonly been documented in the lower limbs, followed by the upper limbs, trunk, and head and neck area. Recently, visceral involvement of the kidney and periampullary region have been described.Reference Lau, Klein, Jiang, Weiss and Chu12, Reference Ramdial, Sing, Deonarain, Singh, Allopi and Moodley15 The age of afflicted patients ranges from 13 to 87 years.Reference McMenamin, Fletcher, Unni and Mertens13 There is a distinct male predilection. Whilst the head and neck region is a recognised site,Reference Datta, Rawal, Mincer and Anderson4, Reference Terada6, Reference Wilson, Hellquist, Ray and Pickles7, Reference Xia, Chen, Geng, Jiang, Yang and Zhang16 there has been no previous report in the English-language literature of myopericytoma involving the external auditory canal. Masses in the external auditory canal pose clinicopathological challenges to otolaryngologists and histopathologists because of the diverse aetiological possibilities and overlapping presentations. Whilst the clinical diagnostic possibilities include congenital and acquired infective, metabolic, genetic and neoplastic diseases, the histopathological assessment narrows this spectrum. The cytoarchitectural hallmark of myopericytomas is the variable concentric perivascular growth of tumour cells with an oval to spindle morphology. The immunohistochemical profile of myopericytoma, which is now better defined, is characterised by tumoural immunopositivity for alpha smooth muscle actin and h-caldesmon.Reference McMenamin, Fletcher, Unni and Mertens13 Desmin may be focally positive or negative.Reference McMenamin, Fletcher, Unni and Mertens13
Despite the known typical pathological features of myopericytoma, diagnostic histopathological challenges arise because of shared phenotypic and immunophenotypic features of benign and malignant tumours in this location (Table I). Infantile myofibroma and myofibromatosis are associated with fibroblastic or myofibroblastic proliferation, and have a biphasic zonation pattern and haemangiopericytomatous vasculature that lacks the concentric perivascular cellular arrangement and h-caldesmon immmunopositivity.Reference Mentzel, Dei Tos, Sapi and Kutzner8, Reference Dray, McCarthy, Palmer, Bonar, Stalley and Marjoniemi11 In contrast to myopericytoma, angioleiomyoma and leiomyoma demonstrate a desmin-immunopositive mature muscle phenotype.Reference Mentzel, Dei Tos, Sapi and Kutzner8, Reference McMenamin, Fletcher, Unni and Mertens13 Whilst spindle cell haemangioma contains spindle cells and blood vessels, the latter are of cavernous morphology.Reference Weiss, Goldblum, Weiss and Goldblum17 The whirling pattern of tumour cells around blood vessels is reminiscent of a meningioma; however, meningiomas are usually epithelial membrane antigen positive, and alpha smooth muscle actin and muscle-specific actin immunonegative.Reference Perry, Louis, Scheithauer, Budka, von Deimling, Louis, Ohgaki, Wiestler and Cavenee18 Myopericytoma, haemangiopericytoma and solitary fibrous tumour demonstrate intratumoural staghorn-type vasculature, but only myopericytoma demonstrates a striking multilayered growth of spindle cells with a myoid immunophenotype around blood vessels. Myopericytoma lacks the CD99 and CD34 immunopositive spindle cells that are typical of haemangiopericytoma,Reference Xia, Chen, Geng, Jiang, Yang and Zhang16 and the cytoarchitectural spectrum and CD34 immunopositive cells of solitary fibrous tumour.Reference Gengler and Guilloi19 Glomus tumours usually lack a spindle cell component and the typical ‘spinning-off’ of tumour cells directly from the blood vessels, as seen in myopericytoma.Reference Mentzel, Dei Tos, Sapi and Kutzner8 Jugulotympanic paraganglioma, a highly vascular tumour, has a typical nest-like growth pattern of variably spindled cells that are synaptophysin and chromogranin immunopositive.Reference Weiss, Goldblum, Weiss and Goldblum17 Kaposi sarcoma, a human herpesvirus 8 positive, spindle cell tumour, demonstrates an endothelial rather than a myopericytic immunophenotype.Reference Lamovec, Knuutila, Fletcher, Unni and Mertens20
• Myopericytoma is a relatively recently described cutaneous and soft tissue tumour
• It presents rarely in the head and neck area, but its occurrence in the ear is undocumented
• It is characterised by a perivascular proliferation of spindle cells, with perivascular myoid cell or pericytic differentiation
• This paper describes a novel case of myopericytoma involving the external auditory canal and tragus
• Heightened clinicopathological awareness of myopericytoma is pivotal to diagnosis
• Myopericytoma needs distinguishing from other benign and malignant tumours with similar features
Table I Histopathological mimickers of head and neck myopericytoma

In conclusion, this report details the hitherto unreported occurrence of a myopericytoma that involved the external auditory canal and tragus. Heightened clinicopathological awareness of the expanding anatomical distribution of myopericytoma is critical to its diagnosis and differentiation from microscopic look-alikes that are site-dependent.Reference Ramdial, Sing, Deonarain, Singh, Allopi and Moodley15 Myopericytoma should be added to the range of external auditory canal neoplasms, especially those characterised by an admixture of spindle cells and a prominence of blood vessels, including those with a haemangiopericytomatous pattern.







