Introduction
Burkholderia pseudomallei is distributed in the soil and water of the tropics, especially in a geographical belt extending from South India to Southeast Asia, Thailand and Northern Australia.Reference Karcher, Zaman, Brewis and Fahmy1 A regular inhabitant of rice paddy fields, this bacterium is transmitted via direct contact, inhalation, inoculation or ingestion.Reference White2 The incubation period is not known. The single most important risk factor for developing severe melioidosis is diabetes mellitus.Reference Karcher, Zaman, Brewis and Fahmy1–Reference Dance, Smith, Aucken and Pitt3 Other risk factors include thalassaemia, kidney disease and occupations at high risk of exposure (e.g. rice paddy farming). Clinically, melioidosis may present with local or systemic symptoms. Mortality rates for systemic disease are very high, but local infection is usually mild, often causing delay in seeking medical attention. Relapsed disease is more frequent with poor compliance, development of antibiotic resistance and immunosuppression.Reference John, Jesudason, Lalitha, Ganesh, Mohandas and Cherian4 Diagnostic confusion with tuberculosis can occur, as both diseases are endemic in the same regions.
Case report
A 40-year-old, diabetic farmer visited our out-patient clinic with a three-week history of a painless, left-sided neck swelling and a foreign body sensation in his throat. He had never received regular diabetic medication. He gave no complaint of hoarseness or dysphagia. He stressed that he was not a smoker and denied alcohol abuse. He could not, however, rule out trauma sustained while working in paddy fields.
On examination, a smooth, well defined, 5 × 3 cm mass of firm to hard consistency and not fixed to underlying muscle or bone was found on the left side of the patient's neck in the region of the level III lymph nodes. Wary of malignancy, we proceeded to full ENT investigation, which revealed a suspicious-looking bulge in the lateral wall of the patient's left pyriform fossa, with no other irregularity or obvious mass.
Computed tomography (CT) of the neck revealed a hypo-dense lesion with surrounding enhancement on contrast, extending from the level of the epiglottis to the C5 vertebral level, involving the strap muscles and causing effacement of the pyriform sinus on the left. Direct, laryngoscope-guided biopsies were taken from the specific area, and the histopathology report ruled out the presence of any malignant cells. Apart from aberrant blood glucose levels, the patient's haematological and biochemical parameters were within normal limits. When the fine needle aspiration cytology report confirmed the absence of any malignant tissue in the neck swelling, it was decided to perform an excision biopsy of the mass.
In the course of surgery, a well encapsulated lesion containing 30 ml of creamy, white pus was found. The pus was carefully drained and the capsule meticulously excised from its attachments to skin and underlying muscle. The area was explored and any further lesions were excluded.
The fibrotic capsule was sent for high power histopathological examination, and a diagnosis of tuberculous abscess was returned. Pus culture revealed the growth of Burkholderia pseudomallei, resistant to aminoglycosides. Significantly, acid-fast bacilli were not detected using standard staining and culture methods.
The patient was given antibiotic cover with intravenous cefuroxime, and his blood glucose levels were kept under control with human insulin. The post-operative period was uneventful. The patient was commenced on oral amoxicillin-clavulanate for the maintenance phase. Anti-tubercular therapy was commenced simultaneously.

Fig. 1 Axial computed tomography neck scan showing a well encapsulated mass.
Discussion
Melioidosis is caused by the Gram-negative, environmental, saprophytic bacterium Burkholderia pseudomallei. It closely resembles the causative agent of glanders, and thus was named ‘pseudoglanders’ for a period.Reference Dance, Smith, Aucken and Pitt3–Reference Cheng and Currie5 The condition was discovered in 1911 in Rangoon by Major Alfred Whitmore of the Indian Medical Service along with C S Krishnasami, and became known as Whitmore's disease.Reference White2
Serological studies in endemic areas have shown that subclinical infection is common.Reference White2 There are many presentations, both acute and chronic. Many infections are initially subclinical but may result in latency and delayed manifestation, even after several decades. Clinical signs and symptoms include septicaemia, cavitating pneumonia, bone and soft tissue infections, disseminated abscesses, mycotic aneurysms, lymphadenitis, and childhood parotitis.Reference White2, Reference Cheng and Currie5 Most patients have an underlying predisposition to infection, especially diabetes, renal disease, alcoholism or thalassaemia; however, in the largest Indian case series, 50 per cent of patients had no traditional risk factors.Reference Jesudason, Anbarasu and John6

Fig. 2 Sagittal computed tomography neck scan, showing the neck mass.

Fig. 3 The patient's neck swelling.
The diagnosis of melioidosis is usually established by culture from sterile sites. Laboratory misidentification is not uncommon, and in fact occurred in the current case because the diagnosis was not considered.Reference Cheng and Currie5 Polymerase chain reaction is an emerging diagnostic tool although not yet extensively validated.Reference Novak, Glass, Gee, Gal, Mayo and Currie7 A definite history of contact with soil may not be elicited as melioidosis can be dormant for many years before becoming acute. A serological test for melioidosis has been developed (using indirect haemagglutination).Reference Cheng and Currie5 Imaging of the abdomen using CT or ultrasound is recommended routinely, as abscesses may not be clinically apparent and may coexist with disease elsewhere.
The treatment of melioidosis is divided into two stages: an intravenous, high intensity stage and an oral, maintenance stage to prevent recurrence.Reference Karcher, Zaman, Brewis and Fahmy1, Reference Dance, Smith, Aucken and Pitt3, Reference Cheng and Currie5 Following surgical drainage of the abscesses, the intravenous, intensive phase is commenced. Intravenous ceftazidime is the current drug of choice for treatment of acute melioidosis.Reference White2, Reference Dance, Smith, Aucken and Pitt3, Reference Cheng and Currie5 Meropenem, imipenem and cefoperazone-sulbactam are also active. Intravenous amoxicillin-clavulanate may be used if none of the above four drugs is available, but it produces inferior outcomes.Reference Cheng and Currie5 Intravenous antibiotics are given for a minimum of 10 to 14 days, and are not usually stopped until the patient's temperature has returned to normal for more than 48 hours. Following treatment of the acute disease, it is recommended that eradication treatment with co-trimoxazole and doxycycline be used for 12 to 20 weeks to reduce the rate of recurrence.Reference Karcher, Zaman, Brewis and Fahmy1, Reference Cheng and Currie5 Co-amoxiclav is an alternative for those patients who are unable to take co-trimoxazole and doxycycline (e.g. pregnant women and children under the age of 12 years).

Fig. 4 Intra-operative photograph showing the abscess cavity containing pus.

Fig. 5 Intra-operative photograph showing dissection of the fibrous capsule of the abscess.
Without access to appropriate antibiotics, the septicaemic form of melioidosis has a mortality rate that exceeds 90 per cent.Reference White2, Reference Cheng and Currie5 With appropriate antibiotics, the mortality rate is about 10 per cent for uncomplicated cases but up to 80 per cent for cases with bacteraemia or severe sepsis. Relapse occurs in 10 to 20 per cent of patients. While molecular studies have established that the majority of recurrences are due to the original infecting strain, a significant proportion of recurrences (perhaps up to a quarter) in endemic areas may be due to reinfection, particularly after two years.Reference Dance, Smith, Aucken and Pitt3 Risk factors include severity of disease (patients with positive blood cultures or multifocal disease have a higher risk of relapse), choice of antibiotic for eradication therapy, poor compliance with eradication therapy and eradication therapy lasting less than eight weeks.Reference White2
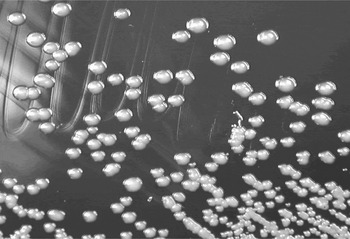
Fig. 6 Burkholderia pseudomallei on culture media.
In endemic areas, people (rice-paddy farmers in particular) are best advised to avoid contact with soil, mud and contaminated surface water where possible. The effectiveness of measures to reduce exposure to the causative organism has not been established. A vaccine is not yet available.Reference Dance8
• This paper describes a case of invasion of a tuberculous cavity by Burkholderia pseudomallei, inoculated via vegetative trauma
• Most patients infected with B pseudomallei have an underlying predisposition to infection, especially diabetes, renal disease or any immunocompromised state
• Increased awareness of melioidosis is important as, although the organism is easy to culture, it may be dismissed as a contaminant
• Intensive treatment with intravenous ceftazidime may make the difference between life and death in patients infected with this rare but potent pathogen
Our patient was unfortunate to suffer from two endemic diseases simultaneously, perhaps representing the first such case in the world literature. In this patient, melioidosis could have been due to super-infection of a tuberculous cavity following vegetative trauma, which would have been an ordinary event in the course of his work in the paddy fields.








