Introduction
Cerebrospinal fluid leakage without an identifiable cause is usually referred to as ‘idiopathic’ or ‘spontaneous’, and accounts for 6–23 per cent of all cerebrospinal fluid fistulas.Reference Schuknecht, Simmen, Briner and Holzmann1 Precise identification of the fistula site and endonasal repair of the osteodural defect is the current treatment of choice.Reference Psaltis, Schlosser, Banks, Yawn and Soler2 This approach reduces the incidence of ascending meningitis, estimated to be 0.12 cases per year for spontaneous leaks and 1.22 cases per year for secondary leaks, unless the leak recurs.Reference Poletti-Muringaseril, Rufibach, Ruef, Holzmann and Soyka3,Reference Bernal-Sprekelsen, Alobid, Mullol, Trobat and Tomas-Barberan4 A high failure rate (25–87 per cent)Reference Wise and Schlosser5 of spontaneous idiopathic cerebrospinal fluid leak closure is attributed to inadequate surgical technique,Reference Castelnuovo, Mauri, Locatelli, Emanuelli, Delu and Giulio6 type of closure,Reference Gassner, Ponikau, Sherris and Kern7 scarring from previous sealing attempts,Reference Wise, Harvey, Neal, Patel, Frankel and Schlosser8 elevated intracranial pressure and morbid obesity.Reference Holzmann and Wild9, Reference Schlosser, Wilensky, Grady, Palmer, Kennedy and Bolger10 The site and size of the defect may be significant.Reference Lindstrom, Toohill, Loehrl and Smith11, Reference Mirza, Thaper, McClelland and Jones12 Closure of sphenoid sinus leaks may be challenging because of limited access and inability to completely remove the mucosa and effectively plug the defect using current endoscopic techniques. The endoscopic trans-pterygoid approach is a promising option; however, surgical expertise is available in only a very few highly specialised centres.Reference Alexander, Chaaban, Riley and Woodworth13 This study aimed to assess the role of intracranial pressure measurement in the closure of spontaneous cerebrospinal fluid leaks. A concise review of evidence supporting the contribution of elevated intracranial pressure to the pathogenesis of cerebrospinal fluid leaks is provided.
Materials and methods
Patients
A retrospective review of the records of patients with spontaneous cerebrospinal fluid leaks of the sphenoid sinus who presented at our tertiary referral centre in a six-year period (2005–2011) was performed. Diagnosis was based on history, clinical, endoscopic and imaging findings. Computed tomography (CT) and magnetic resonance imaging were used to identify the fistula site and the presence of a concomitant meningocele, and to exclude a primary cause for elevated intracranial pressure. Specifically, radiological features of idiopathic intracranial hypertension such as empty sella, lateral sinus collapse, flattened globes and unfolded optic nerve sheaths were investigated. A specific diagnosis of idiopathic intracranial hypertension was made according to the modified Dandy criteria after compiling demographic, clinical, laboratory and imaging data.Reference Smith14 Patients with a discernible cause of intracranial hypertension or fistula were excluded from the study. Laboratory testing with β-trace or β2-transferrin confirmed the presence of an active cerebrospinal fluid leak in all patients. Neurological and ophthalmological consultation completed the assessment.
Intracranial pressure measurements
Intra-operative intracranial pressure measurements were obtained in the operating theatre through a lumbar spinal puncture connected to a manometer, with the patient placed in the lateral decubitus position. A transient increase in intracranial pressure was induced by bilateral pressure on the jugular veins to confirm correct placement. In the case of increased intracranial pressure, a lumbar tap was opened and sufficient cerebrospinal fluid was released (at a rate of 5–10 ml per hour) to reduce cerebrospinal fluid pressure on the graft. The lumbar drain was maintained post-operatively for 2–4 days, and a second intracranial pressure measurement was then taken. If measurements were normal (<2.0 kPa for non-obese patients, <2.4 kPa for overweight patients), the lumbar drain was removed. Patients that consistently showed elevated intracranial pressure or developed hypertension after fistula closure were given a diuretic treatment. Neurosurgical consultation and placement of a ventriculoperitoneal shunt were given in cases of treatment-resistant intracranial hypertension.
Surgical technique
Endoscopic surgery was used for all patients. After preparation of the surgical field with vasoconstrictors, standard sphenoethmoidectomy with preservation of middle turbinates was performed bilaterally. Bleeding from branches of the sphenopalatine and maxillary arteries was controlled by electrocautery. The fistula site was exposed after identification of the cerebrospinal fluid leak, and meningoencephaloceles were cauterised and resected. Bone in proximity to the leak was denuded of mucosa and periosteum. Multilayer closure of the defect was performed in the following order: temporalis fascia or fat was placed through the defect under the dura; a piece of ethmoid bone or septal cartilage was fitted into the defect; and then mucoperiosteum from the middle turbinate was used to cover the area. The three-layer plug was stabilised with fibrin-glue and gelatin sponge or oxidised cellulose, and antibiotic-impregnated gauze or expandable sponge packing was placed in the sphenoid sinus. If access to the lateral recess of the sphenoid sinus precluded direct closure, then the sinus was obliterated with fat. Packing was removed on post-operative day 4 and patients were discharged after 5–8 days and given a one-week course of antibiotics and laxatives. Patients were also instructed to avoid strenuous physical activity for a month. Post-operative follow up for endoscopy and removal of crusting was arranged every week for the first month, after three months and then at regular six-month intervals.
Results
Eleven patients, all primary cases, fulfilled the inclusion criteria and were enrolled in the study. The group comprised eight women and three men, with a mean age of 49.1 years (range 38–61 years), presenting with spontaneous cerebrospinal fluid leaks at a single site in the sphenoid sinus. Patient demographics are shown in Table I.
Table I Patient characteristics and surgical results

*Post-operative measurement. BMI = body mass index; ICP = intracranial pressure; FU = follow up; Y = yes; N = no
Intracranial pressure recordings were available for eight patients: intracranial pressure was elevated in six pre-operatively and in two post-operatively. Papilloedema was noted in one patient and radiological signs of idiopathic intracranial hypertension were present in seven. Two asymptomatic patients with normal weight and imaging findings suffered from elevated intracranial pressure.
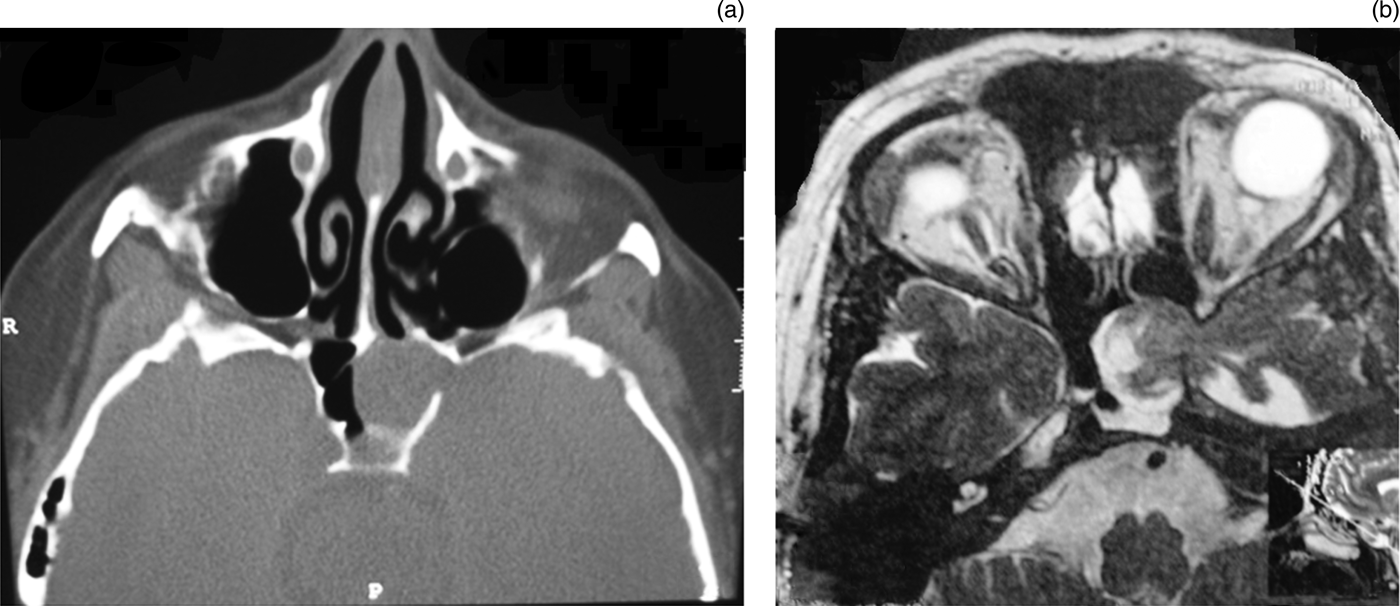
Additional lesions were observed in four patients: an arachnoid cyst in the lateral recess of the sphenoid sinus (in one); a meningoencephalocele (in two); and a meningocele (in one). The exact locations of the fistulas are shown in Figure 1. Figure 2 shows a CT image of a large meningoencephalocele in one patient, and the final post-operative result can be seen in Figure 3. The size of the osteodural defects ranged from 3 to 7 mm. Patient 11 had a defect in the lateral wall of the sphenoid which precluded the use of standard closure with fascia. Instead, a fat plug was inserted into the defect, followed by bone and mucoperiosteum. Specific measures for intracranial pressure reduction (acetazolamide and advice on weight loss) were taken in 5 out of 10 patients with pre- or post-operative intracranial hypertension. Cerebrospinal fluid leakage recurred in one patient within two weeks of surgery. This patient had an intracranial pressure of 3.7 kPa. A ventriculoperitoneal shunt was inserted and the leak was sealed using the same technique without further recurrence in the 67 months until follow up. No major complications were noted. The overall success rate was 90.9 per cent after a mean follow up of 37.1 months (range 8–72 months).

Fig. 1 Image showing cerebrospinal fluid fistula sites in the sphenoid sinus. The anterior walls and intersinus septum have been removed. Stars indicate sites of cerebrospinal fluid leaks in the sphenoid sinus.

Fig. 2 (a) Axial computed tomography scan of the skull base showing a large bone defect on the lateral wall of the left sphenoid sinus which is totally opacified. R = right; P = posterior. (b) Axial magnetic resonance imaging scan of the same patient reveals that the soft tissue mass in the left sinus is a meningoencephalocele of the left temporal lobe.

Fig. 3 Axial magnetic resonance image of the patient shown in Figure 2 three months after successful cerebrospinal fluid leakage closure using fascia, septal cartilage and conchal mucoperiosteum. The left sphenoid sinus was filled with gelatin foam and oxidised cellulose pieces. The whitish soft tissue represents fibrosis.
Discussion
The main finding of our series is that patients with spontaneous cerebrospinal fluid leakage at the sphenoid sinus may suffer from elevated intracranial pressure, for which additional interventions should be considered. Two patients had elevated intracranial pressure without the typical epidemiological, clinical or imaging features of idiopathic intracranial hypertension, further supporting the use of intracranial pressure measurements. One of our first patients, in whom standard closure failed, prompted us to offer invasive intracranial pressure measurement to all patients with a spontaneous peri-operative cerebrospinal fluid leak.
Two factors seem to play a major role in the pathogenesis of spontaneous cerebrospinal fluid leakage in the sphenoid sinus: a congenital or acquired skull base defect and elevated intracranial pressure. Lateral sphenoid defects may be present in up to 9.8 per cent of autopsy specimens from the normal population.Reference Hooper15 Congenital defects, such as lateral craniopharyngeal canal (Sternberg's canal), have been associated with cerebrospinal fluid fistulas of the skull baseReference Schick, Draf, Kahle, Weber and Wallenfang16 but cannot account for most spontaneous cases. In 1888, Sternberg reported the presence of a dehiscent lateral craniopharyngeal canal corresponding to an area medial to the foramen rotundum in 4 per cent of autopsy specimens. Barañano et al. scrutinised the paranasal sinus CT scans of 1000 patients with problems unrelated to cerebrospinal fluid leaks and found only 1 sphenoid defect medial to the foramen rotundum.Reference Barañano, Cure, Palmer and Woodworth17 Schuknecht et al. found that the osseous defect was lateral to the foramen rotundum in eight cases with a sphenoid fistula.Reference Schuknecht, Simmen, Briner and Holzmann1
It is more likely that an osseous defect develops from the combination of aberrant arachnoid granulations (the major cerebrospinal fluid outflow pathway) and an excessively pneumatised and thinned skull base. Thirteen per cent of the autopsy specimens in the normal population show aberrant arachnoid granulations close to the pneumatised areas of the tegmen.Reference Yew, Dubbs, Tong, Nager, Niparko and Tatlipinar18 Arachnoid granulations are known to increase in size with advancing age, possibly as a result of intensified cerebrospinal fluid pulsations in the upright position or during physical activity.Reference Gacek, Gacek and Tart19 Intracranial hypertension accelerates bone resorption around the arachnoid granulations, leading to osseous defects specifically localised to areas of excess pneumatisation.Reference Prichard, Isaacson, Oghalai, Coker and Vrabec20 Clinical and radiographic data support this theory: arachnoid pits (seen as erosions of the skull base) are found in 63–87.5 per cent of patients with a sphenoid cerebrospinal fluid leakReference Schuknecht, Simmen, Briner and Holzmann1, Reference Shetty, Shroff, Fatterpekar, Sahani and Kirtane21, Reference Silver, Moonis, Schlosser, Bolger and Loevner22 and in only 0–23.4 per cent of the normal sphenoid sinuses.Reference Barañano, Cure, Palmer and Woodworth17, Reference Shetty, Shroff, Fatterpekar, Sahani and Kirtane21 Excessively pneumatised sphenoid sinus was reported in 91 per cent of patients with cerebrospinal fluid leakage, but in only 23 per cent of age-matched controls.
Intracranial hypertension has a significant role in the development of spontaneous cerebrospinal fluid fistulas of the paranasal sinuses.Reference Woodworth, Prince, Chiu, Cohen, Schlosser and Bolger23 Cerebrospinal fluid absorption is impaired at the level of the arachnoid granulations and possibly the extracranial lymphatics.Reference Boulton, Armstrong, Flessner, Hay, Szalai and Johnston24 Increased cerebrospinal fluid pressure particularly affects points of inherent weakness such as the lateral recess of an excessively pneumatised sphenoid sinus. Over time, the dura enters the sella turcica or the paranasal sinuses and ruptures under the constant cerebrospinal fluid pressure. Epidemiological, radiological and clinical data support an association between intracranial hypertension and cerebrospinal fluid leaks.
Cerebrospinal fluid fistulas are common in obese middle-aged women with occult or manifest intracranial hypertension.Reference Woodworth, Prince, Chiu, Cohen, Schlosser and Bolger23, Reference Schlosser, Wilensky, Grady and Bolger25–Reference Schlosser, Woodworth, Wilensky, Grady and Bolger27 Obesity is strongly associated with intracranial hypertension,Reference Giuseffi, Wall, Siegel and Rojas28 and weight reduction alone can reduce intracranial pressure and stop spontaneous cerebrospinal fluid leakage. Diet and gastric surgery have proven useful in reducing intracranial pressure and its consequences.Reference Johnson, Krohel, Madsen and March29–Reference Sugerman, Felton, Sismanis, Kellum, DeMaria and Sugerman31 It should be noted that non-obese patients may also suffer from intracranial hypertension, which may go unnoticed if not specifically investigated.
Long-standing intracranial hypertension often precedes the development of a spontaneous fistula. Clark et al. reported four patients with known intracranial hypertension who presented with a spontaneous paranasal sinus fistula after a period of 8 months to 11 years.Reference Clark, Bullock, Hui and Firth32 Increased intracranial pressure has also been associated with lateral skull base spontaneous cerebrospinal fluid leaks. An empty sella, the radiological hallmark of elevated intracranial pressure, is often found in patients with a temporal bone cerebrospinal fluid fistula,Reference Goddard, Meyer, Nguyen and Lambert33 and elevated intracranial pressure has been reported in cases of recurrent or multiple fistulas of the temporal bone.Reference Kari and Mattox34 The presence of fistulas in the anterior, as well as the lateral, skull base implies a single, ongoing process and further supports the theory that intracranial hypertension is a causative factor of cerebrospinal fluid fistulas.
• Elevated intracranial pressure and osseous skull base defects contribute to spontaneous cerebrospinal fluid fistulas in the sphenoid sinus
• Standard three-layer closure may not be feasible for lateral fistulas
• Successful management requires osseous defect closure with a three-layer technique or fat obliteration and intracranial pressure reduction
• Invasive peri-operative intracranial pressure measurement is recommended for patients with overt intracranial hypertension
Despite mounting evidence for a link between intracranial hypertension and cerebrospinal fluid leaks, peri-operative intracranial pressure monitoring and control is not universally adopted. Lumbar puncture is an invasive, uncomfortable procedure which may rarely be complicated by pneumocephalus and meningitis. Several authors have achieved a high long-lasting closure rate without intracranial pressure recording and treatment.Reference Bernal-Sprekelsen, Alobid, Mullol, Trobat and Tomas-Barberan4, Reference Lindstrom, Toohill, Loehrl and Smith11, Reference Castelnuovo, Dallan, Pistochini, Battaglia, Locatelli and Bignami35, Reference Casiano and Jassir36–Reference Kirtane, Lall, Chavan and Satwalekar39 Other authors have found that uncontrolled intracranial pressure and obesity account for the recurrence.Reference Schlosser, Wilensky, Grady, Palmer, Kennedy and Bolger10, Reference Mirza, Thaper, McClelland and Jones12, Reference Cassano and Felippu40–Reference Carrau, Snyderman and Kassam43 Woodworth et al. reported that three patients with a failed cerebrospinal fluid closure had a documented ventriculoperitoneal shunt malfunction, suggesting that elevated intracranial pressure contributed to the recurrence.Reference Woodworth, Prince, Chiu, Cohen, Schlosser and Bolger23 The same group recently reported the outcome of 13 lateral sphenoid cerebrospinal fluid leaks, of which 8 were previous failures.Reference Alexander, Chaaban, Riley and Woodworth13 A success rate of 92 per cent after a median follow up of 10.8 months was achieved using an endoscopic trans-pterygoid approach and intracranial pressure control measures.
Conclusion
Successful closure of sphenoid sinus cerebrospinal fluid leaks requires a three-layer technique incorporating a hard tissue such as bone or cartilage. Elevated intracranial pressure is important in the pathogenesis of spontaneous cerebrospinal fluid leaks of the skull base, and should therefore be identified and treated aggressively. In a few patients with intracranial hypertension, epidemiological features and clinical and radiological signs are absent. We advocate intra- and post-operative intracranial pressure measurement via a lumbar puncture for all patients. This is most important for cerebrospinal fluid leaks in the lateral sphenoid sinus wall. The relative inaccessibility of this area to current endoscopic approaches makes insertion of a bony or septal graft into the osseous defect difficult. A fat plug may not withstand cerebrospinal fluid pulsations, leading to recurrence of the leak if intracranial hypertension is not reduced. Assessment by a multidisciplinary team including neurologists and neurological surgeons is necessary for discussing the available options for controlling intracranial pressure with patients.