Introduction
Hypopharyngeal squamous cell carcinoma (SCC) is a rare entity, and accounts for approximately 3–5 percent of all head and neck SCCs.Reference Hall, Groome, Irish and O'Sullivan1 The hypopharynx extends from the superior border of the hyoid bone to the lower border of the cricoid cartilage, and is a muscle-lined tube extending from the oropharynx to the cervical oesophagus. The World Health Organization (WHO) defines four sites in the hypopharynx: the post-cricoid region, the pyriform sinus, the hypopharyngeal aspect of the aryepiglottic fold and the posterior wall of the hypopharynx.Reference Edge, Byrd, Compton, Fritz, Greene and Trotti2
Approximately 14 400 new cases of pharyngeal SCC are diagnosed each year, with approximately 3400 new cases of hypopharyngeal cancer diagnosed annually.Reference Edge and Compton3 At the time of diagnosis, the majority of the patients have advanced disease with lymph node involvement and a propensity for distant metastasis.Reference Hoffman, Karnell, Shah, Ariyan, Brown and Fee4 Patients are usually not aware of the problem until the tumour is large, and obstructive symptoms or pain occur. Submucosal spread is a characteristic feature, as is direct invasion of adjacent structures in the neck.Reference Pingree, Davis, Reichman and Derrick5–Reference Frank, Garb, Kay, McClish, Bethke and Lind7 A rich network of lymphatics results in frequent neck node metastasis: ipsilateral involvement in 60–80 percent and contralateral occult nodal metastases in up to 40 percent of patients at the time of diagnosis.Reference Takes, Strojan, Silver, Bradley, Haigentz and Wolf8
The five-year survival rates in stage III and IV disease have been reported at only 15–45 percent.Reference Takes, Strojan, Silver, Bradley, Haigentz and Wolf8 A worse prognosis of hypopharyngeal SCC is related to several factors other than lymphatic and systemic spread, such as a predisposition to develop secondary malignancies, frequent association with major alcohol abuse, associated co-morbidities and frequent nutritional depletion.Reference Reis, Aguiar, Alzamora, Ferreira, Castro and Soares9
The traditional treatment approach for locally advanced hypopharyngeal cancer has been laryngopharyngectomy and pharyngeal reconstruction, with or without adjuvant radiotherapy (RT), leading to the loss of natural speech.Reference Bova, Goh, Poulson and Coman10 One reason for this approach was that salvage surgery following the failure of irradiation in these patients was known to cause higher morbidity compared to patients with other head and neck cancers. Larynx preservation had previously been primarily assessed in patients with laryngeal cancer. As patients with hypopharyngeal carcinoma already have a poor overall condition, specialists had been reluctant to explore larynx preservation in this subset.Reference Lefebvre, Chevalier, Luboinski, Kirkpatrick, Collete and Sahmoud11 The necessity for improving survival along with larynx preservation led to chemotherapy being introduced as a new treatment modality in advanced disease.
In the early 1980s in the USA, the Department of Veterans Affairs Laryngeal Cancer Study Group initiated a randomised trial for patients with laryngeal carcinoma, to determine if induction chemotherapy followed by definitive radiation in responders (with surgery reserved for salvage) could be better than the standard treatment of surgery and post-operative radiation.Reference Wolf, Fisher, Hong, Hillman and Spaulding12 In this trial, induction chemotherapy followed by RT in responders offered survival rates comparable to that achieved with laryngectomy followed by RT. In light of these results, chemoradiotherapy has been studied for the last 20 years as a first-line treatment for advanced head and neck cancer patients, including those with hypopharyngeal cancer, which represents a smaller subgroup.Reference Adelstein, Li, Adams, Wagner, Kish and Ensley13–Reference Shin, Glisson, Khuri, Lippman, Ginsberg and Diaz16
Despite the adoption of chemoradiotherapy as a standard of care in these patients, there is no level 1 evidence regarding the best treatment for advanced hypopharyngeal carcinoma.Reference Robson17 In a locally advanced setting, the decision to treat continues to be controversial, and appropriate treatment requires a multidisciplinary approach. This new systematic review aimed to determine if surgical management (radical surgery followed by adjuvant treatment) has a survival advantage compared to treatment with non-surgical modalities, including induction chemotherapy and concurrent chemoradiotherapy, in advanced disease.
Materials and methods
Study types
A systematic review was conducted of randomised controlled trials and retrospective observational studies published from January 1980 to January 2017. Overall survival for surgical and non-surgical treatments in advanced stage hypopharyngeal carcinoma was compared.
Inclusion criteria
All retrospective studies and randomised trials with adult patients (aged 18–75 years), who had a histologically and imaging (computed tomography or magnetic resonance imaging) confirmed diagnosis of advanced stage hypopharynx carcinoma, including selected tumour (T) stage T2 cases (tumour–node stage T2N1 or T2N2), as well as T3 or T4 cases with any nodal (N) stage (N0, N1, N2 or N3) as per the American Joint Committee on Cancer,18 were included in the review. The primary tumour had to be resectable in the first instance.
Intervention types
The treatments included immediate surgery (total laryngectomy, total laryngopharyngectomy, partial pharyngectomy, and partial laryngectomy with partial pharyngectomy), followed by post-operative RT or post-operative concurrent chemoradiotherapy. Non-surgical treatments included induction chemotherapy, followed by RT and concurrent chemoradiotherapy.
Outcomes
The primary outcome assessed was overall survival. Studies reporting a three- or five-year overall survival rate were included. The secondary outcome of the study was larynx preservation rate.
Exclusion criteria
Studies reporting patients with metastatic disease, stage I or II disease, primary tumours at other sites, inoperable disease, prior treatment with RT (complete or incomplete), prior larynx-preserving surgery or prior transoral surgery, were excluded. Only studies published in English were included in the review.
Study identification
A computer-based search of online medical databases was undertaken. The databases used were Medline/PubMed,19 Embase,20 Scopus,21 the Cochrane Library22 and Google Scholar. All databases were searched from 1 January 1980 to 1 January 2017. PubMed and Ovid were the main providers used to develop the search strategy.23
The Medical Subject Heading terms used to develop the search strategy were ‘hypopharyngeal neoplasms’, ‘hypopharyngeal cancer’, ‘hypopharyngeal carcinoma’, ‘hypopharyngeal SCC’, ‘survival rate’, ‘surgery’ and ‘concurrent chemoradiation’. The search strategy used in Medline/PubMed is provided in Appendix 1. Other keywords used were ‘laryngectomy’, ‘laryngopharyngectomy’, ‘postoperative radiotherapy’ and ‘larynx preservation’ in all the databases.
As this review was a part of a post-graduate dissertation, it was not possible to search specific journals or to screen review articles. The first author alone developed and conducted the search.
Study selection
Eligibility assessment of the studies was performed independently and in an unblinded standardised manner by one reviewer. RefWorks online manager software was used to organise the studies.24 Titles were screened for relevance. When relevant titles were found, abstracts were screened, and studies were excluded or included accordingly. When some of the inclusion criteria were noted in the abstract, the full-text articles were examined to assess the rest of the criteria. All stages of the screening were performed by one reviewer. Duplicates were removed prior to data extraction. An inter-library loan service was accessed via the University of Edinburgh for articles that were inaccessible through the university databases. This process is summarised in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (‘PRISMA’) flow chart (Figure 1).Reference Moher, Liberati, Tetzlaff and Altman25

Fig. 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (‘PRISMA’) flow chart.
Data extraction
Data were extracted by one reviewer. Data are reported in table and text format within the review. Data were entered into Microsoft™ Excel software (2013) to generate tables and graphs. Two authors were contacted via e-mail for missing data for two of the studies selected for inclusion in the review.Reference Vandersteen, Benezery, Chamorey, Ettaiche, Dassonville and Poissonnet26, Reference Chang, Wang, Kang, Huang, Lin and Fang27 Unfortunately, the missing data were not provided before this study was completed. The extracted data were rechecked by the primary author to ensure that any errors were corrected. The study authors were not contacted to confirm the accuracy of the data published in their studies.
Data items
For each included study, data on patient characteristics were extracted, including the mean or median age, gender, smoking and alcohol status, disease stage (including tumour and nodal stage), site of the cancer within the hypopharynx, follow-up period, performance status, treatment modalities (surgery followed by adjuvant therapy, concurrent chemoradiotherapy alone, and induction chemotherapy followed by RT), doses of RT and chemotherapy, as well as survival outcomes (including five-year overall survival, three-year overall survival, and, where available, disease-specific survival, disease-free survival and organ preservation rates). The main focus of the study was overall survival.
Quality assessment
The EQUATOR (Enhancing the Quality and Transparency of Health Research) network was accessed to assess the quality of all the included studies.28 Randomised trials were assessed using the Consolidated Standards of Reporting Trials (‘CONSORT’) 2010 checklist.29 The Strengthening the Reporting of Observational Studies in Epidemiology (‘STROBE’) statement was used to assess the quality of observational studies.30
Review Manager software (version 5.3) was utilised to compute a risk of bias summary for each randomised study.31 This figure is provided in Appendix 2. Both assessments were performed independently by the primary author alone.
Summary measures and planned analysis
A meta-analysis was performed for the randomised controlled trials. A fixed-effects model and the generic inverse variance method were used to generate the meta-analysis, as it was assumed that the studies had a common treatment effect. The chi-square test was used to estimate heterogeneity between studies. A forest plot was generated using the Review Manager software to summarise the meta-analysis outcome. The number of events from each randomised study was extracted to generate the meta-analysis. As survival was the primary outcome of the report, hazard ratios with 95 percent confidence intervals (CIs) were calculated. A p value of less than 0.05 was considered statistically significant. Statistical guidance was obtained from the Cochrane Handbook for Systematic Reviews of Interventions.Reference Higgins and Green32
The observational studies were not included in the meta-analysis because this would provide misleading outcomes and inaccurate results. A weighted mean of the overall five-year survival rates for surgical and non-surgical treatments was calculated. Individual study data are presented for studies reporting three-year overall survival rates.
Results
Study selection
The systematic review included 2 randomised controlled trialsReference Lefebvre, Chevalier, Luboinski, Kirkpatrick, Collete and Sahmoud11, Reference Beauvillain, Mahe, Bourdin, Peuvrel, Bergerot and Rivière33 and 11 observational studies.Reference Reis, Aguiar, Alzamora, Ferreira, Castro and Soares9, Reference Vandersteen, Benezery, Chamorey, Ettaiche, Dassonville and Poissonnet26, Reference Chang, Wang, Kang, Huang, Lin and Fang27, Reference Zelefsky, Kraus, Pfister, Raben, Shah and Strong34–Reference Harris, Biron, Donald, Farwell, Luu and Bewley41 A thorough search of PubMed and Medline, Embase, Scopus, the Cochrane Library, and Google Scholar yielded 5639 citations. After removing duplicates, 4839 titles remained. Of these, 4814 were excluded after screening the title alone because they reported on other cancers. Twenty-five studies had relevant abstracts; the full texts of these were examined for the inclusion criteria. Two studies did not separately examine advanced stage disease, one article had a hypopharyngeal cancer sample size that was too small, one study did not provide p values and CIs, and one article included a study on metastatic disease. Seven other studies that were not accessible online were obtained from the Edinburgh University inter-library loan service. Of these, none met the inclusion criteria. No unpublished reports were included. The search is summarised in the flow chart (Figure 1). The list of excluded articles is provided in Appendix 3.
Randomised studies
Methods
Two randomised studies published in English were included in the review.Reference Lefebvre, Chevalier, Luboinski, Kirkpatrick, Collete and Sahmoud11, Reference Beauvillain, Mahe, Bourdin, Peuvrel, Bergerot and Rivière33 In the European Organization for Research and Treatment of Cancer (‘EORTC’) phase III trial, by Lefebvre et al., the median follow-up period was 51 months.Reference Lefebvre, Chevalier, Luboinski, Kirkpatrick, Collete and Sahmoud11 Beauvillain et al. reported a median follow-up period of 92 months. In the latter study, randomisation was always performed prior to RT.Reference Beauvillain, Mahe, Bourdin, Peuvrel, Bergerot and Rivière33
Participants
The 2 trials involved 286 patients in total. There were 276 males and 10 females. The median age in both studies was 55 years. The inclusion criteria were similar for both studies: adults with advanced stage carcinoma of the hypopharynx that was deemed operable in the first instance. Both studies included patients with a WHO performance status of 2 or less.
Interventions
The European Organization for Research and Treatment of Cancer trial patients were randomised to receive either immediate surgery (total laryngectomy with partial pharyngectomy) followed by RT (50–70 Gy), or induction chemotherapy (cisplatin (100 mg/m2)) given as an intravenous bolus injection on day 1, followed by a fluorouracil (1000 mg/m2) infusion on days 1–5. Patients with a complete response after either two or three cycles of chemotherapy were thereafter treated by irradiation (70 Gy), and patients who were non-responders underwent conventional surgery followed by post-operative radiation (50–70 Gy). The type of neck dissection used was determined by the clinical node status: for patients with stage N0, a modified neck dissection was proposed that spared the sternocleidomastoid muscle, the internal jugular vein and the accessory nerve. For those with stage N1 or N2, only the nerve removal was optional. For the N3 patients, a classic radical neck dissection was mandatory.
In the Beauvillain et al. trial, all patients received three courses of neoadjuvant chemotherapy before locoregional treatment, which was randomised between patients undergoing surgery plus post-operative RT, and RT with or without salvage surgery. Randomisation was performed prior to chemotherapy. The chemotherapy consisted of three courses of cisplatin (100 mg/m2) on day 1 and fluorouracil (1 g/m2) on days 2–5, and there was a 1-week interval between courses. Locoregional treatment was administered on day 45. The patients assigned to surgery underwent total laryngopharyngectomy, plus unilateral or bilateral radical or conservative lymph node dissection. The tumour bed received 50–60 Gy, and the involved nodes received 60–70 Gy. The study characteristics are presented in Table I.Reference Lefebvre, Chevalier, Luboinski, Kirkpatrick, Collete and Sahmoud11, Reference Beauvillain, Mahe, Bourdin, Peuvrel, Bergerot and Rivière33
Table I Characteristics of randomised trials

M = males; F = females; SRT = surgery plus radiotherapy; CRT = chemoradiotherapy; post-op = post-operative; RT = radiotherapy
Outcomes
In both randomised controlled trials, the primary outcome assessed was three- or five-year overall survival (Table II).Reference Lefebvre, Chevalier, Luboinski, Kirkpatrick, Collete and Sahmoud11, Reference Beauvillain, Mahe, Bourdin, Peuvrel, Bergerot and Rivière33 The studies described data on the patterns of failure according to treatment groups, which included locoregional control and chemotherapy toxicity. Larynx preservation was discussed in detail in the European Organization for Research and Treatment of Cancer trial.
Table II Overall survival in randomised trials

SRT = surgery plus radiotherapy; CRT = chemoradiotherapy
Risk of bias
The Cochrane collaboration risk of bias assessment table was used to assess the risk of bias using Review Manager software (version 5.3).31 The findings are presented in the form of graphs (Figure 2 and Appendix 2). To summarise, neither study discussed the blinding of patients and personnel, or the blinding of outcome assessment. Figure 3 shows the Consolidated Standards of Reporting Trials score of the randomised trials.

Fig. 2 Risk of bias graph: review of authors' judgements about each risk of bias item presented as percentages across both randomised studies.

Fig. 3 Consolidated Standards of Reporting Trials (‘CONSORT’) score for the randomised studies.
Lefebvre et al
The overall three-year survival rate was 57 percent in the chemoradiotherapy group and 43 percent in the immediate surgery group. The median survival duration was 25 months in the immediate surgery arm and 44 months in the induction chemotherapy arm; there was an observed death hazard ratio of 0.86 in favour of the chemoradiotherapy group. The upper limit of the 99.65 percent CI (a 95 percent corrected CI) of the death hazard ratio reached only 1.43; thus, the two treatments were judged to be equivalent.
The trend for disease-free survival was similar to that for overall survival. The three- and five-year disease-free survival rates were 43 percent (95 percent corrected CI = 28–58 percent) and 25 percent (11 patients at risk) in the chemotherapy arm, versus 32 percent (95 percent corrected CI = 17–47 percent) and 27 percent (8 patients at risk) in the surgery arm, respectively.
In the surgery arm, only two patients did not undergo radical surgery: one patient had general deterioration in health and the other underwent radiation therapy because of node unresectability. This trial presented estimates for larynx preservation in the entire group of 100 patients who received non-surgical treatment. In the European Organization for Research and Treatment of Cancer trial, estimated survival in patients with a functional larynx was 28 percent at three years (95 percent CI = 17–37 percent) and 17 percent at five years (95 percent CI = 8–26 percent). Larynx preservation was also taken into account in patients who died of causes other than local disease progression; the three-and five-year estimates in this group were 42 percent (95 percent CI = 31–53 percent) and 35 percent (95 percent CI = 22–48 percent), respectively. For 52 patients who achieved a complete response in the chemoradiotherapy group, the 3- and 5-year estimates associated with a functional larynx were 64 percent (95 percent CI = 48–80 percent) and 58 percent (95 percent CI = 40–76 percent), respectively. The authors concluded that larynx preservation with induction chemotherapy was safe in the setting of advanced hypopharyngeal cancer. Tumour staging is presented in Table III.Reference Lefebvre, Chevalier, Luboinski, Kirkpatrick, Collete and Sahmoud11, Reference Beauvillain, Mahe, Bourdin, Peuvrel, Bergerot and Rivière33
Table III Tumour staging in randomised trials

Data represent numbers of cases. SRT = surgery plus radiotherapy; CRT = chemoradiotherapy
Beauvillain et al
This trial reported a five-year overall survival rate of 19 percent in the chemoradiotherapy arm versus 37 percent in the surgery arm. Median survival was 40 months in the surgery arm versus 20 months in the chemoradiotherapy arm, which showed a statistically significant difference (p = 0.04). This survival difference was because the surgery arm had a significantly better five-year local control rate (63 percent) than the chemoradiotherapy group (39 percent) (p < 0.01).
A meta-analysis of the two randomised trials was performed to compare overall survival between the patients who received surgery and those who did not (Figure 4).
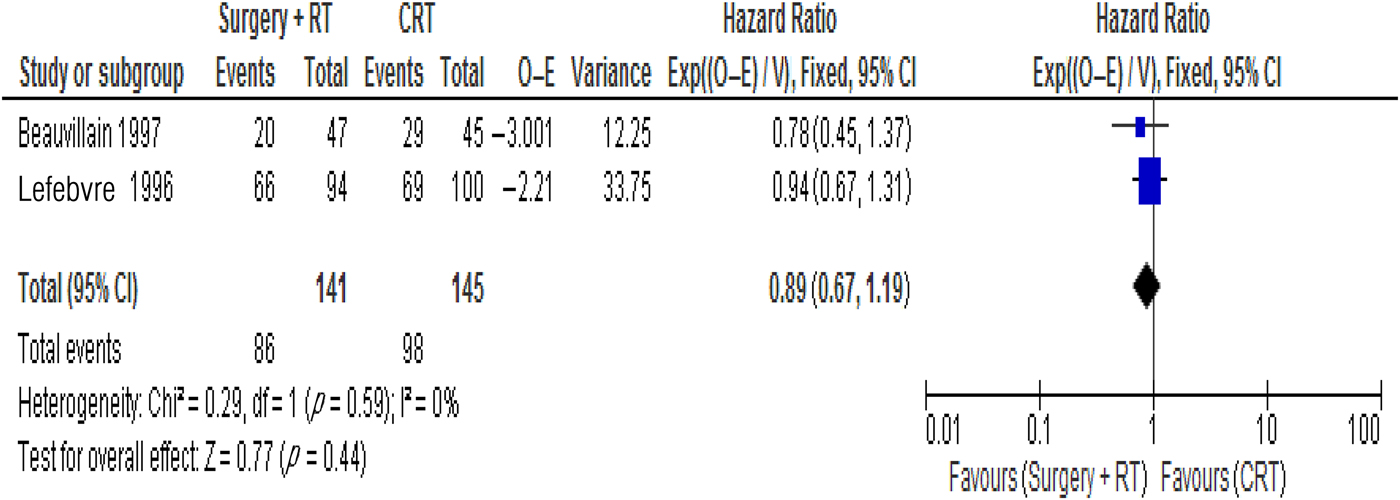
Fig. 4 Forest plot showing the treatment effect in terms of overall survival. RT = radiotherapy; CRT = chemoradiotherapy; O = observed; E = expected; Exp = exponential; V = variance; CI = confidence interval; df = degrees of freedom
Data from the trials showed a hazard ratio of 0.89 favouring surgery (95 percent CI = 0.67–1.19). The test for an overall effect showed a p value of 0.44. Neither treatment showed clearly superior overall survival. The chi-square test assessing heterogeneity between the studies produced a value of 0.29 (p = 0.59). This was not statistically significant. A Cochran's Q test (I2) value of 0 percent further indicated minimal between-study heterogeneity.
Observational studies
Methods
All 11 non-randomised studies were retrospective observational studies that were published in English.Reference Reis, Aguiar, Alzamora, Ferreira, Castro and Soares9, Reference Vandersteen, Benezery, Chamorey, Ettaiche, Dassonville and Poissonnet26, Reference Chang, Wang, Kang, Huang, Lin and Fang27, Reference Zelefsky, Kraus, Pfister, Raben, Shah and Strong34–Reference Harris, Biron, Donald, Farwell, Luu and Bewley41 All studies with the exception of two reported data only on advanced stage hypopharyngeal cancer. The data pertinent to the advanced stage were extracted from these two studies.Reference Chang, Wang, Kang, Huang, Lin and Fang27, Reference Jang, Kim, Cho, Jung, Oh and Ahn36 The Strengthening the Reporting of Observational Studies in Epidemiology (quality) scores for the observational studies are shown in Figure 5.

Fig. 5 Strengthening the Reporting of Observational Studies in Epidemiology (‘STROBE’) scores for the observational studies.
Participants
The included studies had a total of 1320 patients aged 18 years and over. There were 1273 males and 47 females. All participants (Table IVReference Reis, Aguiar, Alzamora, Ferreira, Castro and Soares9, Reference Vandersteen, Benezery, Chamorey, Ettaiche, Dassonville and Poissonnet26, Reference Chang, Wang, Kang, Huang, Lin and Fang27, Reference Zelefsky, Kraus, Pfister, Raben, Shah and Strong34–43) met the inclusion criteria, which required adults with resectable advanced stage hypopharyngeal carcinoma, who received either surgical or non-surgical treatment.
Table IV Patients’ characteristics in observational studies

Interventions
The main interventions included radical surgery (total laryngectomy or total laryngopharyngectomy), followed by adjuvant treatment with either RT, chemotherapy or concurrent chemoradiotherapy. Non-surgical treatments included: induction chemotherapy followed by definitive RT, definitive chemoradiotherapy, concurrent chemoradiotherapy, induction chemotherapy followed by concurrent chemoradiotherapy, and primary RT followed by concurrent chemoradiotherapy.
Primary outcome
In all studies, the primary outcome assessed by the authors was overall survival in relation to treatment modality.
Secondary outcomes
The secondary outcomes included the rate of larynx preservation, as well as factors associated with treatment toxicity and prognosis. One study reported functional outcomes (verbal communication and dysphagia),Reference Jang, Kim, Cho, Jung, Oh and Ahn36 and one study reported outcomes on second primary malignancy.Reference Chang, Wang, Kang, Huang, Lin and Fang27
Treatment and overall survival
Zelefsky et al. reported 30 patients who were treated with surgery and post-operative RT, as well as 26 patients who received induction chemotherapy and definitive RT with laryngectomy reserved for salvage treatment.Reference Zelefsky, Kraus, Pfister, Raben, Shah and Strong34 Surgery involved total laryngectomy in 60 percent of the patients and partial pharyngectomy in the other 40 percent. The main chemotherapeutic agents used were cisplatin and 5-fluorouracil, and some patients were administered cisplatin combined with bleomycin and vinblastine. The median RT dose was 66–72 Gy for the chemoradiotherapy group and 57 Gy in the post-operative patients. The surgically treated patients had an overall five-year survival rate of 22 percent, and patients treated with RT and chemotherapy had an overall five-year survival rate of 15 percent (p = 0.65). A superior outcome was observed in the group receiving surgery; however, this was not statistically significant.
Kim et al. reviewed 91 patients.Reference Kim, Kim, Kim, Kim, Lee and Kim35 Of these, 57 were treated with surgery and post-operative RT; 34 patients received definitive chemoradiotherapy. The types of surgery included: (1) partial laryngectomy with partial pharyngectomy; (2) total laryngectomy and partial pharyngectomy; and (3) total laryngopharyngectomy. The chemotherapy agents included cisplatin, 5-fluorouracil and Taxotere®. The median RT dose was 70 Gy in the chemoradiotherapy group and 59.4 Gy in the surgery group. This study had a median follow-up period of 50 months. The five-year overall survival rate was 58.6 percent for the chemoradiotherapy group and 56.60 percent for the surgery group (p = 0.713).
A Korean study by Jang et al. analysed data from 177 patients with advanced stage hypopharyngeal carcinoma.Reference Jang, Kim, Cho, Jung, Oh and Ahn36 The median follow-up duration for the patients was 19 months. Twenty patients underwent surgery and adjuvant concurrent chemoradiation, 50 patients were treated with initial surgery and adjuvant RT, and 107 patients were treated with induction chemoradiotherapy. The five-year estimated overall survival rates for both the surgical and non-surgical groups were between 45 and 50 percent (log-rank p = 0.991).
Chang et al. published results from 395 hypopharyngeal cancer patients treated with surgery as well as non-surgical organ preservation modalities.Reference Chang, Wang, Kang, Huang, Lin and Fang27 Data were collected from January 1994 to May 2004. Eighty-one patients received radical surgery; the remaining patients received organ preservation intended therapy. The patients in the surgery group with risk factors such as more than two lymph node metastases, positive pathological margins or extracapsular extension also received concomitant chemotherapy when post-operative RT was performed. In the organ preservation intended therapy group, 188 patients received induction chemotherapy followed by RT, 47 patients received RT alone and 79 patients received concurrent chemoradiotherapy. The chemotherapy regimen consisted of oral tegafur, cisplatin and oral leucovorin for 14 days. The RT dose was 60–68.4 Gy in the radical surgery group and 68.4–76 Gy in the organ preservation intended therapy group. The median follow-up duration was 5.09 years. There was no significant difference in overall survival (p = 0.449) between the radical surgery group and organ preservation intended therapy group when the analysis was confined to stage III and IV disease.
Lee et al. presented results from a study comparing concurrent chemoradiation with surgery plus post-operative RT.Reference Lee, Ho, Hsiao, Hwang, Lee and Hung37 The surgery consisted of total laryngectomy with total or partial pharyngectomy, and oesophagectomy with flap reconstruction as indicated. A total of 74 patient records were evaluated (December 1994 to December 2004), with a median follow-up duration of 21 months. Thirty patients received concurrent chemoradiotherapy, and 44 patients received surgery plus RT. There was no significant difference between the T and N staging in both groups. The patients in the concurrent chemoradiotherapy group received 1980–7560 cGy of radiation. Chemotherapy was cisplatin-based. The median survival for these patients was 20 months (range, 3–55 months), with an estimated 3-year overall survival rate of 39 percent. The surgery plus RT group patients received 3420–6660 cGy of radiation. The median survival for this group was 24 months (range, 3–132 months). The estimated three-year overall survival rate was 44 percent. No significant differences were observed in overall survival (p > 0.05).
Hung et al. reviewed the records of 60 patients with advanced hypopharyngeal cancer (stage III and IV) receiving surgical and non-surgical treatments from December 1994 to December 2004.Reference Hung, Chen, Hsieh, Hsu, Chang and Liu38 Thirty-eight patients received definitive concurrent chemoradiotherapy followed by adjuvant systemic chemotherapy; 22 patients were treated with surgery and post-operative concurrent chemoradiotherapy followed by adjuvant systemic chemotherapy. The median follow-up duration at the beginning of the analysis was 20 months. Surgery consisted of total laryngectomy, with total or partial pharyngectomy, and oesophagectomy with flap reconstruction as indicated. The concurrent chemoradiotherapy group was administered 6660–7200 cGy. The surgery and chemoradiotherapy followed by adjuvant systemic chemotherapy group was administered 5940–6660 cGy of RT. Chemotherapy was cisplatin-based. In the surgery and chemoradiotherapy followed by adjuvant systemic chemotherapy group, the mean survival was 23 months. The estimated three-year overall survival rate was 43 percent. The mean survival for patients in the concurrent chemoradiotherapy group was 24 months, and the estimated 3-year overall survival was 38 percent. No significant differences were observed in overall survival between the two groups (p > 0.05).
Another Korean study, by Kim et al., presented results from patients with advanced stage disease treated between August 1979 and July 1997.Reference Kim, Wu, Heo, Kim, Sung and Park39 Seventy-three patient records were analysed, including those who received: (1) RT alone (n = 23); (2) surgery with post-operative RT (n = 18); or (3) neoadjuvant chemotherapy plus RT (n = 32). Surgery consisted of total laryngectomy, partial laryngectomy and pharyngolaryngectomy, and one patient received laser arytenoidectomy. The RT doses were 50–65 Gy in the surgery group and 60.8–73.8 Gy in the sequential chemoradiotherapy group. The median follow-up duration was 28 months. The overall five-year survival rates were 15.7 percent for the RT alone group, 46.8 percent for the surgery plus post-operative RT group, and 43 percent for the neoadjuvant chemotherapy plus RT group. Although both combined treatments (surgery with post-operative RT, and neoadjuvant chemotherapy with RT) were significantly better in terms of overall survival compared to the RT-only group (p = 0.001, p = 0.03, respectively), there was no statistically significant difference between the treatments regarding overall survival (p = 0.15).
Huang et al. performed an observational study in which intensity-modulated RT plus concurrent chemotherapy was used to preserve the larynx.Reference Huang, Jen, Chen, Su, Lin and Lin40 Records from January 2003 to November 2007 were analysed. The median follow-up period was 19.4 months for all patients and 25.8 months for those who remained alive. Fourteen patients were treated with primary surgery with either RT alone or post-operative concurrent chemoradiotherapy, whereas 33 patients received concurrent chemoradiotherapy with the intensity-modulated RT technique. Surgery included total laryngectomy with total pharyngectomy, total laryngectomy with partial pharyngectomy, and neck dissection based on the nodal status. The median RT doses in the surgery and concurrent chemoradiotherapy groups were 62 Gy and 70 Gy, respectively. Chemotherapy was based on cisplatin and 5-fluorouracil. The five-year overall survival rates for primary surgery and concurrent chemoradiotherapy were 33 percent and 44 percent, respectively (p = 0.788).
Harris et al. published results from 76 patients with advanced stage hypopharyngeal carcinoma who were diagnosed between January 1999 and April 2013.Reference Harris, Biron, Donald, Farwell, Luu and Bewley41 Twenty-eight patients underwent primary surgery (total laryngectomy plus partial pharyngectomy with or without regional or free-flap tissue transfer), followed by either RT alone (n = 7) or chemotherapy plus concurrent RT (n = 21) for nodal extracapsular extension (n = 8), or positive surgical margins when re-resection was not achievable (n = 5). The median dose of RT was 65 Gy in the surgery group. A total of 48 patients underwent definitive chemoradiotherapy with or without salvage surgery. The patients receiving definitive chemoradiotherapy received a 70 Gy median dose. The chemotherapeutic agents included cisplatin (n = 17), carboplatin or paclitaxel (n = 6), and cetuximab (n = 6). The details for 12 patients were unavailable. The mean follow-up duration was 30 months, and there were no significant differences between the two groups regarding age, tumour–node–metastasis stage, sex, smoking or alcohol use. The five-year overall survival rate estimate was 66.3 percent in the surgery group and 41.3 percent in the definitive chemoradiotherapy group (p = 0.09). Multivariate analysis showed clinically superior overall survival with up-front surgical treatment compared to definitive chemoradiotherapy (p = 0.06); however, this difference was not statistically significant.
Reis et al. analysed results from 144 patients who received either surgical or non-surgical treatment for locally advanced SCC of the hypopharynx.Reference Reis, Aguiar, Alzamora, Ferreira, Castro and Soares9 A total of 63 patients received surgery followed by adjuvant RT. Of these, 39 patients also received chemotherapy concomitantly with RT, consisting of cisplatin in 37 patients and carboplatin in 2 patients. The median dose of RT in the surgical group was 66 Gy. The other 81 patients received radical RT integrated into an organ preservation approach. The RT doses in this group were 60 Gy and 50 Gy for high- and low-risk regions, respectively. Most patients in this group received chemotherapy concurrently. This consisted of cisplatin in 60 patients, cisplatin plus 5-fluorouracil in 1 patient, and carboplatin in 4 patients. Induction chemotherapy was also administered to 25 patients in the radical RT group. This consisted of cisplatin plus 5-fluorouracil (in 13 patients), or docetaxel plus cisplatin plus 5-fluorouracil (in 12 patients). The median follow-up duration was 36.6 months. The median overall survival for the entire population was 26 months (49 months for stage III patients and 21 months for stage IV patients). The two-year overall survival rates were 55.6 percent for the surgery group and 47.5 percent for the non-surgery group. The five-year overall survival rates were 32.8 percent and 29.2 percent for the surgical and non-surgical treatment groups, respectively (p = 0.274). The treatment regimen did not significantly influence overall survival.
Vandersteen et al. reported survival outcomes in 100 patients with locally advanced hypopharyngeal cancer.Reference Vandersteen, Benezery, Chamorey, Ettaiche, Dassonville and Poissonnet26 The patients were treated from 2001 to 2012. Three treatments were described: (1) induction chemotherapy, followed by either RT with or without chemoradiotherapy, or total pharyngolaryngectomy if there was an absence of response to induction chemotherapy; (2) primary RT, or chemoradiotherapy or cetuximab; and, finally, (3) primary total pharyngolaryngectomy plus post-operative RT, with or without concurrent chemoradiotherapy. Fifty-four patients received induction chemotherapy, including 34 who received docetaxel plus cisplatin and fluorouracil, and 20 who received cisplatin plus fluorouracil. After 2–4 cycles of induction chemotherapy, 24 patients received chemoradiotherapy and 21 patients received RT. Twenty-four patients received primary RT (n = 10) or chemoradiotherapy (n = 14). Twenty patients were treated with total pharyngolaryngectomy plus post-operative RT (n = 13) or concurrent chemoradiotherapy (n = 7). Chemotherapy was cisplatin- and 5-fluorouracil-based. The median follow-up duration was 43 months. The overall three-year survival rate was 58 percent for the group receiving induction chemotherapy, and 47 percent for the group receiving primary RT or chemoradiotherapy. The primary total pharyngolaryngectomy group had a three-year overall survival rate of 36 percent. The p values for the comparison were not presented in the study; however, the authors provided the results of a univariate analysis. This analysis showed that the T stage (T4, p = 0.05) and the American Society of Anesthesiologists Physical Status Classification System43 grade (of more than 3, p = 0.02) were the only significant predictors of overall survival.
To summarise the above results, a weighted mean of overall survival was calculated for surgical and non-surgical treatments. In the eight studies reporting five-year overall survival, an overall survival rate of 44.62 percent was found for patients undergoing surgical treatment with adjuvant therapy, compared to 40.39 percent for patients treated with non-surgical modalities. Individual study data for overall survival are presented in Table V, and the treatments are presented in Table VI.Reference Reis, Aguiar, Alzamora, Ferreira, Castro and Soares9, Reference Vandersteen, Benezery, Chamorey, Ettaiche, Dassonville and Poissonnet26, Reference Chang, Wang, Kang, Huang, Lin and Fang27, Reference Zelefsky, Kraus, Pfister, Raben, Shah and Strong34–Reference Harris, Biron, Donald, Farwell, Luu and Bewley41
Table V Survival data in observational studies

M = males; F = females; N/A = not available
Table VI Treatment modalities in observational studies

Post-op = post-operative; RT = radiotherapy; CRT = chemoradiotherapy; IMRT = intensity-modulated radiotherapy; TPL = total pharyngolaryngectomy
Larynx preservation
Seven observational studies reported data on larynx preservation in advanced disease.Reference Vandersteen, Benezery, Chamorey, Ettaiche, Dassonville and Poissonnet26, Reference Kim, Kim, Kim, Kim, Lee and Kim35–Reference Huang, Jen, Chen, Su, Lin and Lin40 Kim et al. obtained a neoadjuvant chemotherapy response rate of 75 percent (24 out of 32 patients).Reference Kim, Wu, Heo, Kim, Sung and Park39 Overall, 12 patients (38 percent) retained their larynx for longer than 5 years in the neoadjuvant chemotherapy plus RT group. One of these patients had a tracheostomy, but the others did not have a tracheostomy, gastrostomy or feeding tube. Thus, a functioning larynx was retained in 11 patients (34 percent).
In the study by Lee et al., 26 patients completed concurrent chemoradiotherapy, 12 had complete remission, 8 had partial remission and 6 had persistent disease.Reference Lee, Ho, Hsiao, Hwang, Lee and Hung37 The response rate to concurrent chemoradiotherapy was 77 percent (20 out of 26 patients). Eleven patients underwent salvage treatment because of residual or recurrent disease after concurrent chemoradiotherapy or adjuvant chemotherapy. Salvage treatment was successful in five patients. Eight patients (27 percent) retained their laryngeal function for more than two years in the concurrent chemoradiotherapy group.
Huang et al. reported results following definitive concurrent chemoradiotherapy.Reference Huang, Jen, Chen, Su, Lin and Lin40 In this group, six patients underwent salvage surgery (one neck dissection, and five laryngectomy with neck dissection procedures). Eventually, 22 patients had a preserved functional larynx. The five-year functional larynx-preservation survival and laryngectomy-free survival rates were 40 percent and 43 percent, respectively.
Jang et al. reported larynx preservation in terms of the functional outcomes of patients with advanced stage disease.Reference Jang, Kim, Cho, Jung, Oh and Ahn36 After laryngeal resection, 60 percent of the patients in the surgery group retained their ability to speak, in comparison with 76.6 percent of the non-surgically treated patients (p = 0.008). The patients in the surgery group showed worse laryngectomy-free survival rates than those in the initial chemoradiotherapy group. The requirement for additional surgery after initial treatment was similar among both groups (28–28.6 percent). Salvage surgical procedures were more frequent in the chemoradiotherapy group (21.5 percent) than in the surgery group (10 percent p = 0.046).
Kim et al. reported results from 57 patients in the surgery group, in which 17 retained a functional larynx by avoiding total laryngectomy.Reference Kim, Kim, Kim, Kim, Lee and Kim35 This produced a laryngeal function preservation rate of 29.8 percent. Thirty of 34 patients in the chemoradiotherapy group retained a functional larynx (88.2 percent). The loss of laryngeal function in four patients from the chemoradiotherapy group was a result of local recurrence.
Vandersteen et al. reported functional outcomes in all patients treated with the three modalities.Reference Vandersteen, Benezery, Chamorey, Ettaiche, Dassonville and Poissonnet26 The dysphagia outcomes were not different in any of the treatment modalities. None of the patients who received larynx-preserving therapy required tracheostomy. At 1 and 2 years, 33 and 23 patients, respectively (42 percent and 29 percent), were alive, disease-free and with a functional larynx.
Hung et al. reported 34 patients who completed concurrent chemoradiotherapy.Reference Hung, Chen, Hsieh, Hsu, Chang and Liu38 Eight patients achieved complete remission, 18 had partial remission and 8 showed persistent disease. The response rate to concurrent chemoradiotherapy was 77 percent. Fourteen patients underwent salvage intensity-modulated RT because of residual or recurrent disease after concurrent chemoradiotherapy or adjuvant chemotherapy. Ten patients (26 percent) retained a functional larynx for more than two years in the concurrent chemoradiotherapy group.
Discussion
The current evidence is not sufficient to determine the optimum treatment for advanced hypopharyngeal cancer. The randomised study by the European Organization for Research and Treatment of Cancer and observational studies suggests that both radical surgery followed by adjuvant treatment, and a combination of chemotherapy and RT, carried out for advanced stage hypopharyngeal carcinoma, offer similar overall survival.Reference Lefebvre, Chevalier, Luboinski, Kirkpatrick, Collete and Sahmoud11 However, Beauvillain et al. reported a survival advantage with primary chemotherapy followed by radical surgery and post-operative RT, compared to neoadjuvant non-surgical treatment alone.Reference Beauvillain, Mahe, Bourdin, Peuvrel, Bergerot and Rivière33
Following the above randomised studies, treatment evolution has occurred in the field of head and neck cancer, as can be observed in the various combination treatments used in the observational studies within this review. Several phase III trials comparing induction chemotherapy followed by locoregional control with locoregional treatment alone have shown mixed results in terms of reducing distant metastasis and improving survival.Reference Argiris44–Reference Pignon, Bourhis, Domenge and Designe46 A meta-analysis of induction chemotherapy resulted in a non-significant survival improvement of 2 percent at five years (hazard ratio = 0.95, 95 percent CI = 0.8–1.01; p = 0.10).Reference Pignon, Bourhis, Domenge and Designe46 However, there was a significant benefit from cisplatin plus 5-fluorouracil compared with other induction regimens (hazard ratio = 0.88, 95 percent CI = 0.79–0.97). Induction chemotherapy has developed with the introduction of agents containing taxane. Three phase III clinical trials have demonstrated improved survival when a taxane is added to cisplatin plus 5-fluorouracil induction chemotherapy, and is followed by RT or concurrent chemotherapy and RT.Reference Hitt, Lopez-Pousa, Martinez-Trufero, Escrig, Carles and Rizo47–Reference Vermorken, Remenar, Herpen, Gorlia, Mesia and Degardin49
Concurrent chemoradiation was developed as a treatment for head and neck cancer following a meta-analysis.Reference Pignon, Bourhis, Domenge and Designe46 The purpose of this meta-analysis was to evaluate the effectiveness of various chemotherapy timing methods in the management of head and neck SCC. This meta-analysis included 63 trials and 11 000 patients with cancers of the oral cavity, oropharynx, larynx and hypopharynx. The findings demonstrated that the addition of chemotherapy to locoregional treatment conferred an absolute survival benefit of 4 percent at five years (hazard ratio = 0.90, 95 percent CI = 0.85–0.94; p < 0.0001). Chemotherapy given concomitantly showed significant benefits.
These results were tested within the Radiation Therapy Oncology Group 91-11 trial, a three-arm trial comparing different organ-preserving approaches in glottic or supraglottic laryngeal cancer.Reference Forastiere, Goepfert, Maor, Pajak, Weber and Morrison50 The treatment arms were: induction chemotherapy with cisplatin and 5-fluorouracil, followed by RT; concurrent chemoradiation with cisplatin; and RT alone. This trial suggested the superiority of concomitant treatment in terms of larynx preservation and locoregional control. There was no difference in survival rates.
Trials specifically investigating hypopharyngeal carcinoma are limited. Prades et al. reported on the results of a phase III trial comparing concurrent chemoradiotherapy with induction chemotherapy followed by definitive RT.Reference Prades, Lallemant, Garrel, Reyt, Righini and Schmitt51 This trial involved only the tumours of the pyriform sinus with hemilaryngeal fixation. The primary end point was larynx preservation, which was significantly better in the concurrent chemoradiotherapy group, with no difference in survival between the two treatments.
Concurrent chemoradiotherapy has also been applied in the post-operative setting in high-risk patients.Reference Bernier, Vermorken and Koch52 Two phase III trials compared post-operative RT with or without concurrent chemotherapy in patients with high-risk head and neck SCC.Reference Bernier, Domenge, Ozsahin, Matuszewska, Lefebvre and Greiner53, Reference Cooper, Pajak, Forastiere, Jacobs, Campbell and Saxman54 The first study was conducted by the Radiation Therapy Oncology Group, and the second study was conducted by the European Organization for Research and Treatment of Cancer. Both studies concluded that the addition of cisplatin-based chemotherapy improved locoregional control and disease-free survival.
The above findings have markedly increased the use of concurrent chemoradiotherapy in treating head and neck cancers.
Future studies need to evaluate the role of concurrent chemoradiotherapy or taxane-based induction chemotherapy, compared to radical surgery plus concurrent chemoradiotherapy, in terms of oncological outcomes. This would ideally be in the form of a randomised controlled trial comparing the above newer treatments in patients with advanced operable hypopharyngeal cancer. There still appears to be a role for induction chemotherapy, as trials in the 1980s suggested that a response to initial chemotherapy predicted a response to subsequent treatment; additionally, responders had significantly higher survival than non-responders.Reference Ensley, Crissman, Kish, Jacobs, Weaver and Kinzie55–Reference Schuller, Wilson, Smith, Batley and James57 This concept was also cited in the Veterans Affairs Laryngeal Cancer Study. Two major phase III trials comparing taxane-based induction chemotherapy with cisplatin-based concurrent chemoradiotherapy were not able to establish a significant survival advantage for either treatment in locally advanced head and neck cancer.Reference Cohen, Karrison and Kocherginsky58, Reference Haddad, O'Neill and Rabinowits59 In terms of preserving a functional larynx, there is a clear advantage with non-surgical treatments.
Study limitations
The current review has numerous limitations. Regarding the randomised studies, neither study reported blinding for participants, personnel or the outcome assessment, which increased the risk of performance and detection bias. The sample size of the Beauvillain et al. study was small and included less than half the number of patients in the European Organization for Research and Treatment of Cancer trial, suggesting that the Beauvillain et al. study was underpowered.Reference Lefebvre, Chevalier, Luboinski, Kirkpatrick, Collete and Sahmoud11, Reference Beauvillain, Mahe, Bourdin, Peuvrel, Bergerot and Rivière33 This discrepancy could mean that the survival advantage shown in the surgery arm may have been overestimated or underestimated.
The meta-analysis performed in this study estimated the effect of treatment types on overall survival with more precision. The main limitation of the meta-analysis is that the treatment regimens were different in both studies; this is because induction chemotherapy was given to the patients who underwent radical surgery in the study by Beauvillain et al.Reference Beauvillain, Mahe, Bourdin, Peuvrel, Bergerot and Rivière33 They suggested that the poorer outcome with non-surgical treatment in their study could have been related to chemotherapy selection of cells resistant to subsequent RT, thereby resulting in poorer local control.Reference De Pooter, Scalliet, Elst, Huybrechts, Gheuens and Oosterom60
The non-randomised studies were observational and retrospective. As there was no randomisation between the treatment strategies, selection bias is very likely. Furthermore, there was heterogeneity in terms of the type and doses of RT, and in the types of chemotherapy delivered. Most of the studies had a small sample size, with only 3 of the 11 observational studies having a sample size of greater than 100 patients. The modest sample sizes may have overestimated or underestimated treatment effects. Data presented on larynx preservation were limited. Four studies did not report any data regarding organ preservation. These data could not be obtained prior to the completion of this review. Despite this, the rates of larynx preservation are in line with current literature.
Further limitations of the review include the language restriction, as only studies published in English were included. The primary author alone screened the selected studies and performed the quality assessment. There were numerous studies excluded from the current review because of: missing data, a lack of separation of outcomes between early and advanced stage disease, and a lack of data on chemotherapy. Despite the above limitations, the current investigation included studies that reported on advanced stage hypopharyngeal cancer, and patient characteristics were similar across all studies. The primary outcome was overall survival, which was well reported. This systematic review also summarises the latest evidence available on advanced disease following a robust literature search.
Conclusion
All but one of the studies showed that non-surgical organ-sparing treatment had no significant impact on overall survival in advanced hypopharyngeal cancer when compared to the conventional treatment of radical surgery followed by adjuvant treatment. Despite the lack of sufficient evidence in the field of hypopharyngeal cancer, non-surgical treatment for patients with advanced disease is effective because it does not appear to compromise survival compared to surgical treatments. Non-surgical treatment offers an alternative to patients who are unfit for or refuse surgery. Furthermore, there appears to be an opportunity to preserve laryngeal function. The prognosis in advanced disease is poor, and further trials comparing concurrent chemoradiation with conventional surgery and adjuvant treatment are needed.
Acknowledgements
I would like to thank Mr Nigel Beasley, Department of ENT, Sheffield Teaching Hospitals NHS Foundation Trust, for his advice and guidance on this paper, and Elizbeth Hendron (research librarian, Nottingham University Hospitals NHS Trust), for developing the search strategy and confirming the adequacy of the literature search.
Appendix 1. Medline/PubMed strategy

Appendix 2. Risk of bias summary: review authors' judgements about each risk of bias item for each randomised study

Appendix 3. Excluded studies with reasons for exclusion
















