Introduction
Frontal sinus surgery is a challenging undertaking, due to the limited surgical access and the potential for post-operative stenosis of the frontal sinus outflow tract. In the early post-operative phase (two to three days after surgery), fibroblasts form a fibrin mesh, resulting in granulation tissue on which collagen is deposited and scar tissue is formed. In the following weeks, as post-operative inflammation and swelling ensue, areas of the frontal sinus ostium with epithelial damage may make contact. This can lead to adhesions, which may occlude the frontal sinus outflow tract. During the remodelling stage (three weeks after surgery), further scar formation occurs, resulting in concentric narrowing of the frontal sinus outflow tract.Reference Hoyt1
In some patients, instrumentation of the frontal recess results in scarring, synechiae or osteogenesis with re-occlusion of the frontal outflow tract. The fronto-nasal transition zone is particularly prone to scarring, as it is touched frequently during surgery and it is a small, well defined area where contact surfaces are in close proximity. Post-operative scarring can occur in up to 15 per cent of sinus surgery patients, with blockage of the middle meatus leading to recurrent sinusitis in 7 per cent of patients.Reference Shikani2 Endoscopic ethmoidectomy can result in scarring of the frontal sinus outflow tract, and this may be a factor in the observed increased incidence of frontal sinus disease. Re-occlusion is associated with: the diameter of the frontal neo-ostium, polyposis, excessive denuded bone, remnants of osteitic bone in the frontal recess, severe mucosal disease, lateralisation of the middle turbinate, and excessive removal of the middle turbinate.Reference Rains3 Scarring of the nasofrontal duct and post-operative synechiae are associated with endoscopic sinus surgery to the ethmoid sinuses. Therefore, the incidence of iatrogenic chronic frontal sinus disease may rise with increasing use of endoscopic surgery.Reference Benoit and Duncavage4
Frontal sinus stents made from a wide variety of materials have been used to prevent frontal sinus outflow tract re-occlusion and to maintain patency. Using a canine model, Neel et al. showed that a soft Silastic® stent caused less scar formation than when no stent was used.Reference Neel, Whicker and Lake5 To reduce the risk of stenosis following frontal sinusotomy, Rains advised removing agger nasi cell walls and any osteitic bone.Reference Rains3 In addition, this author advised the removal of any anterior ethmoidal cells together with mid-frontal supra-orbital cells, and the preservation of the middle turbinate; the importance of mucosal preservation was also emphasised.
Frontal sinus stenting
Frontal sinus stenting was first reported over 100 years ago.Reference Shikani2 The use of frontal sinus stenting offers a method of draining the frontal sinus and maintaining the frontal outflow tract without radical surgery. The stent prevents scar tissue formation across the frontal sinus opening, and instead allows epithelialisation to occur along the surface of the stent. This process encourages mucosalisation of the frontal sinus outflow tract. Weber et al. prospectively compared the long-term results of patients with and without stents, and found a patent frontal outflow tract in 80 per cent of stented patients, compared with 33 per cent of unstented patients.Reference Weber, Hosemann, Draf, Keerl, Schick and Schinzel6
External approach to frontal sinus stenting
Lynch described the removal of part of the sinus floor and intersinus septum, the sino-orbital wall, and the sinus mucosa through a large opening in the lacrimal bone, before placing a firm rubber tube in the newly created nasofrontal duct. The stent was left in place for five days, but was reported to have a 30 per cent long-term failure rate.Reference Lynch7 Failures with the Lynch procedure were attributed to the removal of the lateral wall, leading to medial collapse of soft tissue and re-stenosis of the nasofrontal drainage pathway. Neel et al., Barton, Yamasoba et al. and Amble et al. have all reported the use of a modified Lynch technique with various materials inserted to stent the frontal sinus, resulting in much improved re-stenosis rates.Reference Neel, Whicker and Lake5, Reference Barton8–Reference Amble, Kern and Neel10 Neel and colleagues preserved the mucosa of the frontal sinus outflow tract and used a soft, thin silicone sheet rolled into a tube as a stent, in patients in whom less bone and mucosa were removed; they reported a 20 per cent failure rate over a five to 20 year follow-up period. Failure of the modified Lynch procedure was again attributed to excessive lateral wall removal, allowing medial collapse of soft tissue and obstruction of the frontal sinus outflow tract.Reference Rains3
Endoscopic insertion of frontal sinus stents
Ingals was the first to describe the endonasal placement of a gold tube frontal sinus stent, in 1905.Reference Shikani2 Schaefer and Close reported the first use of a thin Silastic stent placed endoscopically in the frontal sinus for a period of six weeks.Reference Schaefer and Close11 Weber et al. reported the use of the Rains self-retaining silicone tube, the U-shaped silicone tube and the H-shaped silicone tube for up to six months following surgery.Reference Weber, Mai, Hosemann, Draf and Toffel12 Freeman and Blom described endoscopic placement of a bi-flanged silicone tube in 55 sinuses.Reference Freeman and Blom13 Rains described the endoscopic placement of a soft, self-retaining silicone tube with a collapsible bulb end in 67 patients (102 stents).Reference Rains3
Benoit and Duncavage described a combined external and endoscopic frontal sinusotomy with stent placement.Reference Benoit and Duncavage4 An opening was created in the anterior wall of the frontal sinus with a 4 mm burr. The stent was then placed and pulled down into the nasal cavity using an endoscope.
Gross et al. and Draf both described an endoscopic version of the Lothrop procedure.Reference Gross, Gross, Becker, Moore and Phillips14, Reference Draf15 A large common outflow tract draining both frontal sinuses was created by excising the frontal inter-sinus septum and reducing the upper part of the nasal septum, to create a median drainage pathway, without the need for stenting.
Hoyt described the insertion of a ventilation tube into the frontal sinus, which was secured to the nasal septum with a Vicryl suture for an average of 8.3 weeks.Reference Hoyt1 This procedure was advocated for acute and chronic frontal sinusitis in patients who had undergone at least one previous operation, or who had florid frontal sinus pathology on computed tomography (CT) scanning.
Indications for frontal sinus stenting
There is no agreed consensus on the indications for post-operative frontal sinus stenting. Assessment of a patient's need for frontal sinus stenting should be based on their risk of re-stenosis.Reference Seth J, Joseph B, Richard, Kountakis, Senior and Draf16 There are a number of potential factors that the surgeon should consider: (1) the size of the frontal sinus outflow tract (a neo-ostium diameter of less than 5 mm doubles the rate of re-stenosis (i.e. 16 vs 33 per cent), while a diameter of less than 2 mm increases the rate to 50 per cent); (2) extensive polyposis (as in allergic fungal sinusitis); (3) extensive demucosalisation of the frontal sinus outflow tract, with circumferentially exposed bone; (4) revision frontal sinus surgery with pre-operative scarring; (5) osteitic bone in the frontal recess (determined by pre-operative CT); (6) a flail middle turbinate (particularly when the middle turbinate has been partially resected or lateralised); and (7) traumatic fracture of the frontal sinus outflow tract.Reference Hosemann, Kuhnel, Held, Wagner and Felderhoff17
Careful endoscopic examination should be performed to evaluate the frontal recess for polyposis and scarring from previous nasal surgery. It is also imperative that high definition, fine cut CT scans should be carefully reviewed pre-operatively to evaluate the need for a frontal sinus stent. The diameter of the neo-ostium (formed by the frontal beak, anterior skull base, medial orbit and cribriform plate) should be assessed. Any evidence of allergic fungal sinusitis or osteitis of the frontal sinus outflow tract should be detected by careful review of the CT.
Operative procedure
Direct endoscopic access to the frontal sinus is possible in less than 50 per cent of patients. Such access requires an uncinectomy together with removal of the anterior insertion of the middle turbinate and the agger nasi cells. This improves visualisation and reduces the angle of stent insertion. Any obstructing bone formation, scar tissue or hypertrophic disease should be removed, but care must be taken not to destabilise the middle turbinate. The frontal outflow tract can be expanded by drilling or curetting the bone anteriorly. The choice of stent type and size depends on the size of the neo-ostium. The stent is placed by sliding it over an up-curved frontal sinus suction tube: the bulb tip of the stent is mounted on the end of the suction tube, which is used as an introducer. The inferior end of the stent should not extend below the inferior border of the middle turbinate, and should be trimmed if necessary. The bulb end of the stent is expanded to allow it to be retained in the frontal sinus. The frontal sinus can be irrigated via the stent.
Most studies have used subjective improvement or resolution of frontal sinus symptoms as the main outcome measure. Benoit and Duncavage performed a combined external and endoscopic frontal sinusotomy with stent placement, and recorded the patency of the nasofrontal duct when viewing the opening and transilluminating the frontal sinus using a 30° endoscope intranasally.Reference Benoit and Duncavage4 They reported data for 40 patients: nine patients failed to improve (seven of whom had a patent nasofrontal duct on endoscopic examination and transillumination), while nine patients did not have a patent nasofrontal duct (allergic rhinitis was the most common co-morbidity for all nine). Casiano and Livingston attributed nasofrontal re-stenosis and complete occlusion to a narrowed antero-posterior diameter of the frontal sinus; appropriate patient selection for frontal sinus stent procedures, and evaluation of co-morbidity, was therefore essential.Reference Casiano and Livingston18 Some patients will continue to experience symptoms of frontal sinusitis because of poor mucociliary flow.
Types of frontal sinus stent
Several different types of frontal sinus stent have been used, of various sizes, shapes, materials and consistencies, with varying success. These include gold, tantalum foil, polyethylene teraphthalate (Dacron®) and polymeric silicone (Silastic) sheeting. The Freeman frontal sinus stent is a bi-flanged, 20 mm long, hollow silicone tube available in 14 and 16 Fr diameters.Reference Freeman and Blom13 The Rains silicone stent is widely used, and has a compressible basket at the distal end which can re-expand to assist maintenance of position in situ. This stent can be used as an irrigation port, and is easy to insert endoscopically (being made of soft, malleable silicone rubber). Rains reported a 94 per cent patency rate over eight to 46 months' follow up.Reference Rains3
In an animal model comparing rigid plastic tubes with pliable Silastic sheeting, the softer materials promoted re-epithelialisation and decreased osteoblastic activity and scar formation.Reference Neel, Whicker and Lake5 This was thought to be due to local ischaemia, impaired drainage and infection around the rigid tubes.
Hosemann et al. highlighted the fact that the frontal sinus is particularly anatomically inaccessible to post-operative topical steroids, and that although frontal stents have been shown to be very beneficial, no study has shown them to be 100 per cent effective.Reference Hosemann, Wigand, Gode, Langer and Dunker19 These authors developed a frontal stent made of a polymer that released locally a sustained amount of steroid and antibiotic, in an attempt to improve the success of stenting. This innovation was based on the observation that wound healing in the paranasal sinuses following major sinus surgery may take up to three months.
A recent study by Beule et al. used an animal model to investigate the effect of dexamethasone-releasing stents on wound healing.Reference Beule, Sharf, Biebler, Gopferich, Steinmeier and Wolf20 The drug-releasing stents were found to significantly reduce the level of macroscopic granulation at the wound sites, and the stroma was found to be thinner on the dexamethasone-treated side, compared with silicone stenting. There was no other difference between the two sides, and all wounds healed fully.
Case reports
Patient one
A 46-year-old man with hepatitis C presented with mucoid nasal discharge, left-sided frontal headaches and mouth breathing, associated with nasal speech and hyposmia.
On anterior rhinoscopy, the patient's nasal septum was deviated to the right, with inferior turbinate hypertrophy. Computed tomography (CT) scanning revealed chronic sinusitis and a left frontal osteoma obstructing the frontal sinus outflow tract (Figure 1).

Fig. 1 Pre-operative, coronal computed tomography scan showing osteoma and opaque left frontal sinus.
A combined endoscopic and external approach was attempted, resulting in partial removal of the left frontal osteoma. The patient was subsequently scheduled for a bi-coronal osteoplastic flap procedure with removal of the osteoma but without frontal sinus obliteration. However, further CT scanning showed an aerated left frontal sinus with minimal frontal sinus disease; the procedure was thus cancelled and conservative management continued.
The patient's symptoms of left-sided headache recurred a year later, and further surgery was undertaken. An osteoplastic flap of the left frontal sinus was created, with removal of the osteoma and insertion of a Rains self-retaining frontal sinus stent (Medtronic, Jacksonville, Florida, USA). This stent was left longer than the originally intended six months, to allow the patient to receive treatment for his hepatitis C.
At last follow up, the patient remained asymptomatic. His stent had been in situ for 48 months, and had required no surgical intervention during that period (Figures 2 and 3).

Fig. 2 Post-operative, axial computed tomography scan showing stent in situ.

Fig. 3 Post-operative, coronal computed tomography scan showing stent in situ.
Patient two
A 69-year-old man presented with a painful right eye, head aches and proptosis. He was a heavy smoker and suffered from coronary artery disease. Magnetic resonance imaging (Figure 4) showed a right fronto-ethmoidal mucocele with bony erosion of the medial and lateral borders of the right frontal sinus, confirmed on CT scanning (Figures 5 and 6).

Fig. 4 Patient two: pre-operative, axial magnetic resonance image.

Fig. 5 Patient two: pre-operative, axial computed tomography scan showing right fronto-ethmoidal mucocele.

Fig. 6 Patient two: pre-stent, coronal computed tomography image showing fronto-ethoidal mucocele.
The mucocele was drained using a direct endoscopic approach.
A month later, the patient developed re-stenosis of the right frontal ostium, with recurrence of his symptoms. The right frontal sinus was re-entered endoscopically, with re-opening of the mucocele cavity (which had closed off, with regrowth of membranous mucosa). A self-retaining Rains frontal sinus stent (Medtronic) was inserted; CT scanning confirmed that the stent was in situ (Figures 7 to 9). Because of the patient's significant cardiac co-morbidity, a decision was made to retain the stent on a long-term basis.

Fig. 7 Patient two: post-operative, coronal computed tomography showing stent in situ. H = head; R = right; L = left; F = feet
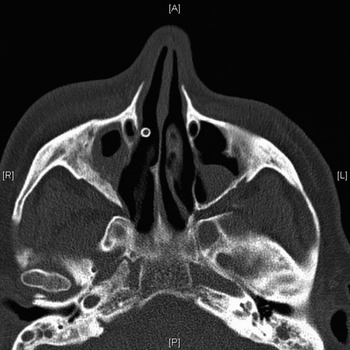
Fig. 8 Patient two: post-operative, axial computed tomography scan showing stent in situ. A = anterior; R = right; L = left; P = posterior

Fig. 9 Patient two: post-operative, sagittal computed tomography scan showing stent in situ. H = head; A = anterior; P = posterior; F = feet
On post-operative follow up, the patient remained asymptomatic, with no eye symptoms and a full range of eye movements. His stent remained patent and in situ for 29 months, before he developed further right-sided headaches. The frontal sinus was re-explored surgically, revealing a patent frontal sinus opening and the stent lying free in the sinus. The stent was replaced. The patient remained symptom-free under regular follow up for a further 19 months.
Patient three
A 92-year-old woman presented with a two-month history of a painful swelling at the medial side of her right orbit.
Computed tomography scanning revealed a large, right fronto-ethmoidal mucocele, with destruction of both the anterior and posterior walls of the frontal sinus. Magnetic resonance imaging confirmed the presence of the mucocele, with bony defects anterior, posterior and inferior to the mucocele. The posterior margin of the mucocele was seen to indent the frontal lobe, with no evidence of invasion into the parenchyma (Figures 10 and 11).

Fig. 10 Pre-operative, axial magnetic resonance image showing left-sided fronto-ethmoidal mucocele.

Fig. 11 Pre-operative, coronal computed tomography scan showing the extent of sinus disease.
A fronto-ethmoidectomy was performed using a combined external and endoscopic approach.
Five months later, the patient developed a tender soft tissue swelling above her right eye. Computed tomography scanning confirmed recurrence of the right frontal mucocele.
The patient underwent re-exploration of her right frontal sinus. A right middle meatal antrostomy was performed via a combined external and intranasal approach, with insertion of a Rains frontal sinus stent (Medtronic).
The stent was removed three months later, and the patient made good clinical progress.
However, later that year the patient complained of a sensation of increasing tension over her right frontal sinus. Computed tomography scanning showed further recurrence of the right frontal mucocele. The mucocele was re-explored via a combined approach, and another Rains self-retaining frontal sinus stent (Medtronic) was inserted. Subsequent to this procedure, the patient complained of epistaxis; the stent was thus changed to a smaller size, with a resulting improvement in the patient's epistaxis symptoms?
The patient was reviewed regularly, and remained free of frontal mucocele symptoms until her death from unrelated causes, over 60 months after stent insertion.
Discussion
Chronic frontal sinusitis is a challenge to treat, due to an often narrow and tortuous frontal sinus outflow tract, limited surgical access, complicated anatomy, and frequent re-stenosis. Successful endoscopic frontal sinus surgery relies on maintaining patency of the frontal sinus outflow tract by preserving the outflow tract mucosa. The fronto-nasal transition zone is a small, well defined area with walls in close proximity to each other, and is very prone to scarring following instrumentation. The frontal sinus is encased by orbital bone, skull base bone and hard frontal bone; therefore, drilling out the frontal sinus outflow tract requires technical skill and experience.
Given the small calibre of the frontal sinus outflow tract and its susceptibility to re-stenosis, complex frontal sinus surgical approaches have been developed, such as the Lynch procedure and its various modifications (e.g. the Draf type III or modified Lothrop procedure, in which a median drainage pathway is created with a widened common outflow tract from both frontal sinuses). These are extensive, technically challenging procedures, with inherent complications and increased patient morbidity. The use of an indwelling frontal sinus stent offers a safe alternative to such complex procedures.
Post-operative care is important in maintaining frontal sinus stent patency. Recommendations include douching with saline three times daily to minimise blockage from dried mucus, long-term topical steroids, and regular out-patient review for observation and debridement. These measures are intended to maintain stent patency and minimise scarring and adhesions, and thus improve long-term results.Reference Lin and Witterick21 Routine debridement is performed in the out-patient department, and uses an endoscope to remove blood clots, mucous plugs, debris and granulation tissue from within the nasal cavity and the stent. In cases of marked polypoidal disease, topical and/or oral steroids will reduce post-operative inflammation and scar formation, and nasal irrigation and douching should be continued while the stent remains in place. Some surgeons also recommend antibiotics for one to two weeks post-operatively.
It is difficult to glean any meaningful data regarding the most desirable duration of stent placement. Different authors have used different stents made from different materials, complicating comparison of post-operative re-stenosis rates. Authors have also reported varying stent placement periods, ranging from 5 days to 17 years.Reference Lynch7, Reference Baron, Dedo and Henry22 Freeman and Blom kept stents in situ for six to 12 months when treating re-stenosis, but only for four weeks to prevent stenosis after a primary surgical procedure.Reference Freeman and Blom13 One study found that frontal sinus stents left in situ for more than six months were more effective than stents removed earlier, and recommended that this be done in difficult revision cases and before performing an external procedure.Reference Hoyt1 The optimal stenting duration will leave the stent in place until wound healing and scar remodelling are complete, with adequate re-epithelialisation and absence of purulent discharge and polypoid mucosa. Due to the increased incidence of steroid-resistant mucosal disease in allergic fungal sinusitis patients, stents should be left in place longer in such cases. Using a canine model stented with soft silicone, Neel et al. found re-epithelialisation of the nasofrontal communication to be complete within eight weeks.Reference Neel, Whicker and Lake5
More recently, long-term stenting has been reported. Orlandi and Knight described nine patients with frontal sinus stents left in situ for 11 to 73 months.Reference Orlandi and Knight23 Only two patients required stent removal, one at 11 months for pain, the other at 61 months due to infection. The other seven patients remained asymptomatic.
Lin and Witterick described a series of 11 patients reviewed over a six-year period.Reference Lin and Witterick21 A total of 21 frontal sinus stents were inserted in that period. Seven patients underwent unilateral stenting, the remainder bilateral. Ten stents in seven patients remained in situ at the end of the study period. Patients remained symptom-free, but four stents needed changing. The remaining 11 stents fell out or were removed at an average of 16.3 months post-operatively. Three stents fell out (14 per cent) at an average of 8.8 months post-operatively, but recurrent symptoms necessitated stent re-insertion. A further eight stents were removed for a variety of reasons: partial dislodgement, blockage, transferral at the time of surgery to the contralateral side, or the finding of a patent, healed frontal recess at follow up. Only one complication was reported: in one patient a stent migrated superiorly and required removal under general anaesthesia. One patient failed treatment and went on to have further frontal sinus surgery.
Frontal sinus stenting is an accepted means of managing chronic frontal sinusitis. It allows ventilation and drainage of the frontal sinus, but cannot prevent the recurrence of polyps. The technique is not without some risk. If the stent is too short, granulation tissue can form across it, embedding it into the ensuing scar tissue. A stent that is too long will cause crusting of the nasal end, which will cause nasal congestion and an unpleasant odour. Displacement of the stent may occur, leading to treatment failure and possible aspiration. Acute frontal sinusitis can occur and requires antibiotic treatment. Chronic rhinosinusitis has been reported in patients with extended placements of stents. The stent may act as a foreign body, and will require removal if medical management fails. There have been descriptions of frontal sinuses stenosing in the frontal recess region, despite stents being in place.
• Long-term frontal sinus stenting may be considered for patients with aggressive disease when re-stenosis occurs, or for patients with co-morbidity in whom complex procedures are ill-advised
• The reported three cases illustrate the success of this approach in patients with hepatitis C, ischaemic heart disease and advanced age
• While frontal sinus stents may require a degree of out-patient care and occasional surgical replacement, they avoid the need for a major surgical procedure
• Due to the high incidence of post-operative frontal sinus outflow tract re-stenosis, and the relatively high failure rates for both endonasal and external frontal sinus surgery, long-term stenting is a useful option in carefully selected patients
Long-term stents may provide a hospitable environment for the formation of bacterial biofilms, which can potentially hinder re-epithelialisation of the frontal outflow tract. A bacterial biofilm comprises an extracellular, polysaccharide matrix that is resistant to host defences and antibiotic therapy. Perloff and Palmer analysed stents placed for one to four weeks in six patients, and found that all stents had bacterial biofilms.Reference Perloff and Palmer24
One case report implicated a frontal sinus stent in toxic shock syndrome after endoscopic sinus surgery.Reference Chadwell, Gustafson and Tami25
Due to these difficulties, we do not recommend the routine use of long-term stents for frontal sinus disease.
Conclusion
Long-term frontal sinus stents may be considered for patients with aggressive disease when recurrent re-stenosis occurs, or for patients with other co-morbidities where multiple complex procedures are ill advised. This is illustrated by our case series of patients who suffered with hepatitis C, ischaemic heart disease and very advanced age. While frontal sinus stents may require a degree of out-patient care and occasional surgical intervention to replace them, they can avoid a major surgical procedure. Due to the propensity for postoperative re-stenosis to the frontal sinus outflow tract and failure of either an endonasal or external frontal sinus operation, long-term stenting remains a useful option in some carefully selected patients.













