Introduction
Recurrent epistaxis is one of the most common presentations to the paediatric ENT clinic. This condition affects 30 per cent of children aged zero to five years, 56 per cent of those aged six to 10 years and 64 per cent of those aged 11–15 yearsReference Petruson1. The aetiology is thought to be multifactorial and may include digital trauma, vestibulitis and crusting.Reference Guarisco and Graham2 Nasal colonisation with Staphylococcus aureus is also thought to play a role, and this has obvious relevance to treatment with antiseptic cream.Reference Whymark, Crampsey, Fraser, Moore, Williams and Kubba3
The relative efficacy of various treatments for recurrent childhood epistaxis has been investigated in numerous studies. Evidence from randomised, controlled trials shows that (1) chlorhexidine–neomycin cream (Naseptin®, Alliance Pharmaceuticals Limited, Chippenham, Wiltshire, UK) is more effective than no treatment,Reference Kubba, MacAndie, Botma, Robison, O'Donnell and Robertson4 (2) chlorhexidine–neomycin cream is as effective as cautery alone,Reference Ruddy, Proops and Pearman5 (3) chlorhexidine–neomycin cream is as effective as combination treatment with cautery and chlorhexidine–neomycin,Reference Murthy, Nilssen, Rao and McClymont6 and (4) petroleum jelly is no more effective than simple observation.Reference Loughran, Spinou, Clement, Cathcart, Kubba and Geddes7
However, these studies all assessed the short-term outcome of individual treatments (follow-up range four to eight weeks). The aim of the current study was to determine the long-term outcome for children treated for recurrent epistaxis, and to compare the relative efficacy of the various treatments received.
Materials and methods
2001 trial
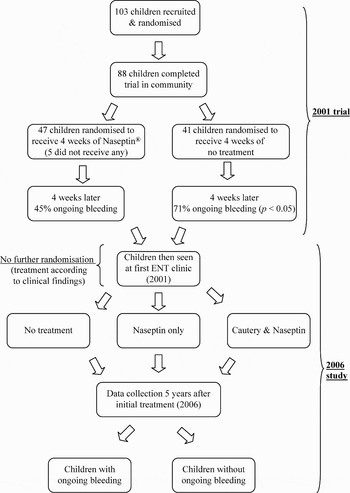
In 2001, 103 children with recurrent epistaxis were recruited into a single-blinded, randomised, controlled trial at our secondary care paediatric otolaryngology centre.Reference Kubba, MacAndie, Botma, Robison, O'Donnell and Robertson4 In summary, the children were randomised to receive a four-week course of chlorhexidine–neomycin cream prior to the first clinic visit (see Figure 1). Eighty-eight children completed the trial, and the frequency of bleeding in the four weeks preceding clinic review was recorded at the first clinic visit. The children who had used chlorhexidine–neomycin cream for four weeks prior to the first clinic attendance were significantly more likely to have complete resolution of their epistaxis, compared with children receiving no treatment.Reference Kubba, MacAndie, Botma, Robison, O'Donnell and Robertson4

Fig. 1 Children's clinical pathway, 2001–2006.
Five-year follow-up study
The purpose of the current study was to report the five-year outcomes of the children who had participated in the original trial, including any treatments they had received over that time. Data on current epistaxis frequency, current and previous chlorhexidine–neomycin cream usage, previous treatment with nasal cautery, and emergency room attendance were collected by telephone interview between June and September 2006. Retrospective case record review was conducted to confirm the treatments received during this period. The hospital's computer system was accessed to confirm otolaryngology out-patient clinic and accident and emergency department (A&E) attendance. Chi-square testing was performed to compare bleeding rates between treatment groups, both on an intention-to-treat and a treatment-received basis.
Ethical considerations
Ethical approval to collect the five-year follow-up data was obtained from the local research ethics committee. Children and parents were each sent information sheets detailing the nature and purpose of the new study, before being contacted by telephone.
Results and analysis
The five-year follow-up study group comprised the 88 children who had completed the original randomised, controlled trial (RCT) in 2001. The children's clinical pathway, through the original RCT and into the current study, is shown in Figure 1. Telephone contact was made with 60 of the 88 children (68 per cent). Details of the treatment received at the first out-patients clinic attendance were obtained from clinical records for 80 of the 88 children. The number of out-patients clinic and A&E attendances was obtained for all 88 children, either from the hospital's computer system or by case note review.
Intention-to-treat analysis
In the original 2001 RCT, 47 children had been randomised to receive four weeks of chlorhexidine–neomycin cream prior to their first clinic attendance. Forty-one children had been randomised to receive no treatment. Of those randomised to receive chlorhexidine–neomycin, five had not used the cream. No child randomised to the control group had received cream inadvertently. The ongoing bleeding rate for each group is shown in Table I. There was no difference between the ongoing bleeding rate of either group (chi-square = 0.018; p = 0.89).
Table I 2006 bleeding rates (intention-to-treat analysis)

* Chlorhexidine–neomycin.
Treatment-received analysis
Children had been treated at the first 2001 clinic visit as shown in Table II. Seven children had undergone cautery under general anaesthetic following their first clinic visit. All children attending the clinic more than once had undergone nasal cautery at least once. In the five-year period following the initial clinic review, seven children had received further chlorhexidine–neomycin cream from their general practitioner to treat recurrent epistaxis.
Table II Treatments received at 2001 clinic visit

* Total n = 80. †Chlorhexidine–neomycin.
Children were divided into three groups according to the treatments they had received during the five-year period between studies, as follows: (1) those receiving no treatment at any time; (2) those receiving chlorhexidine–neomycin cream only; and (3) those receiving cautery and chlorhexidine–neomycin cream. As shown in Table III, the group receiving cautery and cream had the highest ongoing bleeding rate, at five years (chi-square = 4.655; two degrees of freedom; p = 0.098). The children's bleeding frequencies are shown in Figure 2.

Fig. 2 Children's reported bleeding frequencies, 2006.
Table III 2006 bleeding rates of contactable children*: treatment-received analysis

* Total n = 60. †Total n = 39. ‡Chlorhexidine–neomycin.
Out-patients clinic and accident and emergency department attendances
Only five children had attended A&E with epistaxis during the five years. All of these children had been treated with cautery and chlorhexidine–neomycin at some stage. As shown in Figure 3, 62 children (70 per cent) had been discharged after their first clinic visit.

Fig. 3 Children's reported number of clinic visits, 2006.
Referral back to ENT clinic
Eight children had been referred back to the ENT clinic from primary care during the five-year period for treatment of ongoing bleeding.
Discussion
Relevance of study to epistaxis literature
The treatment of recurrent childhood epistaxis, using antiseptic cream with or without nasal cautery, is known to be effective in the short term.Reference Kubba, MacAndie, Botma, Robison, O'Donnell and Robertson4–Reference Murthy, Nilssen, Rao and McClymont6 However, there is no published evidence evaluating the long-term effectiveness of any treatment for recurrent childhood epistaxis. This study demonstrates that the majority of children treated at our clinic had ongoing bleeding after five years.
Clinical applicability of study
Although it is likely that the children's bleeding frequency was less in 2006 than at original presentation in 2001, 49 per cent of the children with ongoing bleeding still had epistaxis at least once a month (see Figure 2). Despite this, only a minority had received further treatment in the community or referral back to our otolaryngology service. The majority of children with ongoing bleeding had received no further treatment; our assumption is that parental tolerance, reassurance that the condition is benign and first aid measures are the mainstays of treatment for the condition.
The fact that children who received cautery and chlorhexidine–neomycin cream had the highest ongoing bleeding rate can be interpreted in different ways. The high bleeding rate in this group does not provide evidence that cautery is ineffective. It may simply reflect the fact that this specific group comprised children with the most persistent bleeding. The finding that all children who had attended the clinic more than once underwent cautery at some stage supports this interpretation.
Study weaknesses
Twenty-eight of the 88 children (32 per cent) in this study were not contactable by telephone, and therefore data collection on current epistaxis frequency was incomplete. We feel that this does not add significant bias to this study. We were unable to contact these children because they had changed their address or contact details since 2001 without informing their previous general practitioner or local general practice registration office. It seems unlikely that a change in domestic circumstances would have any effect on epistaxis frequency, and therefore the bleeding data collected from the 60 contactable children are likely to be representative. Nearly two-thirds of the children who were contactable were still having ongoing epistaxis. It therefore seems unlikely that a higher rate of follow up would change the principal conclusion of this study (that the majority of children treated at our clinic still had ongoing epistaxis after five years).
• This paper highlights the lack of clinical evidence surrounding the long-term efficacy of common treatments for recurrent childhood epistaxis
• Five-year follow-up data were collected on a cohort of children treated for recurrent epistaxis
• Despite incomplete follow up, it was clear that the majority of children treated still had ongoing epistaxis, five years later
• The efficacy of repeated courses of nasal antiseptic cream over an extended time period must be the subject of future epistaxis research
Another criticism of this study is that the method of data collection for current epistaxis frequency was inaccurate and subject to bias. Whilst we accept that an individual's memory of bleeding frequency is of unknown accuracy, we feel that we used the only practical way of collecting such data, and we are unaware of a better or more reliable objective method.
Future paediatric epistaxis research
The role of Staphylococcus aureus nasal colonisation in septal neovascularisation and crusting remains controversial. Our hypothesis is that repeated colonisation is the cause of ongoing bleeding, and therefore repeated courses of chlorhexidine–neomycin cream may be required. This hypothesis remains unproven but must be the subject of paediatric epistaxis research for the future.