Introduction
Laryngeal dysplasia represents part of a spectrum of mucosal changes, characterised by a variable clinical course and a propensity for squamous cell carcinoma (SCC) development. Efforts have been directed towards correlating histopathological grade to clinical outcomes, particularly conversion to malignancy. A meta-analysis of 940 patients in 2010 reported an overall mean transformation rate from dysplasia to invasive carcinoma of 14.0 per cent.Reference Weller, Nankivell, McConkey, Paleri and Mehanna1 The histopathological grade of dysplasia clearly correlates with malignant transformation rates, with the risk increasing three-fold between mild or moderate (10.6 per cent) and severe or carcinoma in situ (CIS) (30.4 per cent) cases.Reference Weller, Nankivell, McConkey, Paleri and Mehanna1 The mean time to transformation is approximately 5.8 years, but there is considerable worldwide variation.Reference Weller, Nankivell, McConkey, Paleri and Mehanna1–Reference Leemans, Braakhuis and Brakenhoff5
Although laryngeal dysplasia represents a series of mutational changes occurring within the overlying stratified squamous epithelium in the larynx,Reference Levendoski, Leydon and Thibeault4 there is a paucity of evidence to demonstrate that sequential epithelial changes occur in a linear fashion.Reference Leemans, Braakhuis and Brakenhoff5 The progression to laryngeal carcinoma has been shown to relate directly to cigarette smoking, alcohol abuse and exposure to other extrinsic irritants that trigger pathways of genetic events. Cigarette smoking and heavy alcohol consumption are both independent and synergistic risk factors for laryngeal cancer.Reference Talamini, Bosetti, La Vecchia, Maso L, Levi and Bidoli6 Despite the conclusive evidence demonstrating the role of human papilloma virus (HPV) in the aetiopathogenesis of oropharyngeal cancer, the significance of this infection in laryngeal cancer needs further evaluation,Reference Halec, Holzinger, Schmitt, Flechtenmacher, Dyckhoff and Lloveras7,Reference Westra8 with studies suggesting that the prevalence of high-risk HPV genotypes in laryngeal SCC shows considerable variation, ranging from 5 to 60 per cent.Reference Pagliuca, Martellucci, Degener, Pierangeli, Greco and Fusconi9–Reference Li, Gao, Li, Gao, Yang and Zhou11
In 2017, the World Health Organization (WHO) updated the classification system of laryngeal dysplasia into a two-tier system, low and high grade, in view of reported inter- and intra-observer variability and lack of standardisation.Reference El-Naggar, Chan, Grandis, Takata and Slootweg12–Reference Cho and Song14 A study by Sannino et al. demonstrated that rates of progression to carcinoma for mild and moderate grades of laryngeal dysplasia were 7.7 per cent and 19.8 per cent, respectively.Reference Sannino, Mehlum, Grøntved, Kjaergaard, Kiss and Godballe15 This raised concerns that the treatment and need for follow up of the newly termed ‘low-grade lesions’ would be crudely underestimated.
The survival rate for non-metastasised laryngeal carcinoma is 90 per cent, reducing to approximately 63 per cent in cases with dissemination to regional lymph nodes, with minimal improvement in these figures over time despite the introduction of novel treatment options.Reference Mozet and Dietz16 A consensus statement by UK ENT surgeons in 2010 outlined the management of the first presentation of laryngeal dysplasia. Cold steel or carbon dioxide (CO2) laser is recommended in the first instance, with radiotherapy reserved for small numbers in rare circumstances such as poor access for resection in a high-grade lesion.Reference Mehanna, Paleri, Robson, Wight and Helliwell17
Given that dysplasia is regarded as a precursor to invasive malignancy, early recognition and characterisation of these lesions could represent a potentially curative stage of the disease. With this in mind, a treatment dilemma exists: oncological safety needs to be balanced with functional outcomes.Reference Karatayli-Ozgursoy, Pacheco-Lopez, Hillel, Best, Bishop and Akst18
Aim and hypothesis
This study aimed to identify whether patients with a histopathological diagnosis of high-grade laryngeal dysplasia have a higher rate of progression to carcinoma compared to patients with low-grade dysplasia during a follow-up period of 10 years at a single tertiary ENT department.
Materials and methods
A retrospective cohort review was conducted of all patients with a histopathological diagnosis of any grade of dysplasia (mild, moderate, severe and CIS), according to the WHO 2005 classification system, affecting the glottis area of the larynx, over a 10-year period from 1 January 2000 to 31 December 2009.
The histopathology database was trawled for the key words of dysplasia affecting the ‘vocal cords’ or ‘larynx’. Data were retrieved by a computer-assisted search of the Systematized Nomenclature of Medicine classification codes for the specific sites of the vocal folds, larynx and laryngeal commissures (classification codes: T24100, T24400, T24410, T24420 and T24471). We identified all grades of laryngeal dysplasia, including those specimens coded as ‘not otherwise specified’, affecting this anatomical region (classification codes: M74000, M74001, M74002, M74003, M74410, M74412 and M80102).
Outcome measures
Patient records were reviewed, and the following data were collected using a pre-defined proforma with Microsoft Excel® spreadsheet software: patient demographics, including age, gender and smoking status at time of diagnosis (smoker, ex-smoker, never smoked, unknown); histopathological findings of laryngeal biopsy, anatomical site and the specific grade of dysplasia; time to diagnosis of SCC from dysplasia during a 10-year follow-up period; and treatment modality for dysplasia (CO2 laser, cold steel excision, radiotherapy, ‘watch and wait’ or other). We also recorded the tumour (T) stage, prior and follow-up biopsy results, 10-year all-cause mortality rates, and requirement for total laryngectomy.
In order to ensure the validity of the results, the primary author reviewed each pathology report on the LabCentre laboratory information management system to confirm accurate coding and consistency of data collection. For cases with multiple grades of dysplasia, the most severe grade was included for analysis. We have categorised severe dysplasia and CIS together as ‘severe’ dysplasia in an attempt to standardise our results with other major studies, including the UK laryngeal dysplasia consensus statement. We have not included hyperplasia or hyperkeratosis cases. Mortality rates are described as either related or unrelated to the underlying laryngeal pathology.
Exclusion criteria
The exclusion criteria were: the presence of simultaneous pharyngeal or laryngeal carcinoma not confined to the glottis; prior laryngeal carcinoma or previous head and neck radiotherapy before the time of the initial biopsy; inappropriately or wrongfully coded sites; and patients with a subsequent diagnosis of SCC within three months, with the rationale that a tissue sampling error may have prevented the identification of invasive carcinoma.
Ethical considerations
This study constituted a retrospective review of local practice; therefore, formal ethical committee approval was not required. This study was registered with the local standards quality and audit department.
Statistical analysis
Data were entered into IBM SPSS® Statistics software, version 26, where all statistical analyses were performed. A Shapiro–Wilk test was conducted to assess normality, in order to determine whether a parametric or non-parametric test should be conducted. Pearson chi-square tests were conducted on parametric categorical variables, and a Fisher's exact test was used for non-parametric categorical variables. Results with a p-value lower than 0.05 were considered statistically significant.
A logistic regression analysis was used to ascertain the effect of dysplasia severity on the likelihood of malignant transformation. A Kaplan–Meier curve was constructed, and a log rank test was performed to determine whether there were differences in the mean length of time to malignant transformation (in months) for different grades of dysplasia.
Results
Data for a total of 317 biopsies from 177 patients were included. Fifty-two patients had a histopathological diagnosis of SCC based on their primary biopsy, leaving 125 patients with any severity of dysplasia (Figure 1). Of the 125 patients with laryngeal dysplasia, the mean average age was 62.8 years (median of 62 years, interquartile range = 56–73 years). The male to female ratio was 2.7:1, including 91 male and 34 female patients.

Fig. 1. Flowchart demonstrating the selection of eligible patients for analysis. SCC = squamous cell carcinoma; CIS = carcinoma in situ; NOS = not otherwise specified
Table 1 outlines the patient demographics of this cohort analysed according to gender. Despite a larger number of male patients within this sample, there was no difference between the mean and median ages of the two groups. The graphical representation of age appears normally distributed with few major outliers.
Table 1. Demographics of patients diagnosed with dysplasia on index biopsy according to gender

IQR = interquartile range
Following the exclusion of 6 patients who underwent ‘rapid progression’ to carcinoma within three months, our analysis examined the remaining 119 patients. Two cases are described as dysplasia ‘not otherwise specified’, as the severity was not specified in the pathology report. The severity of dysplasia is displayed in Figure 2.
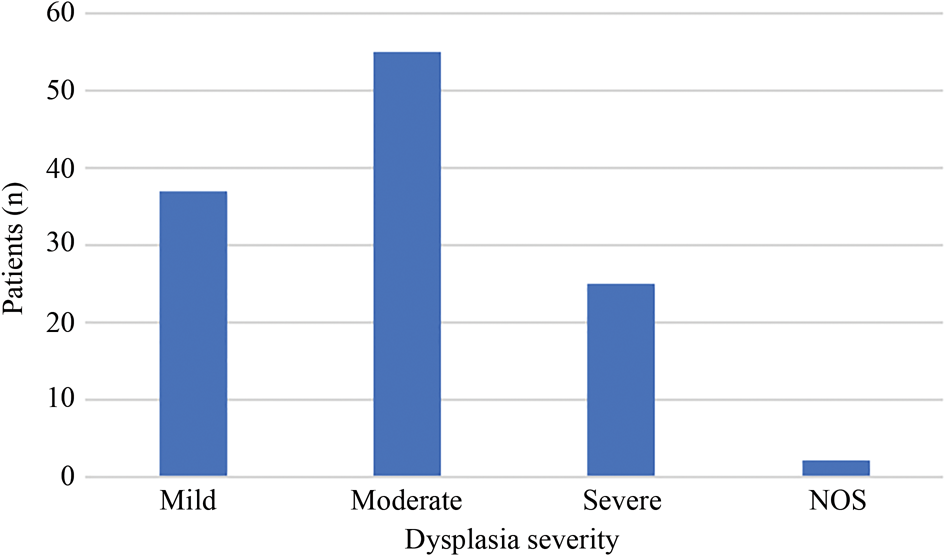
Fig. 2. Distribution of dysplasia cases of differing severity. NOS = not otherwise specified
The overall malignant transformation rate from dysplasia to invasive SCC was 21.8 per cent. This represented 26 of all 119 dysplasia cases. When analysing the effect of histological grade, the malignant transformation rate over 10 years was found to be 10.8 per cent for mild dysplasia, 24.0 per cent for moderate dysplasia and 34.6 per cent for severe dysplasia. The mean time to transformation was 52 months, with the time to conversion reducing with increasing dysplasia severity, as shown in Table 2.
Table 2. Distribution of all dysplasia cases undergoing malignant transformation

Both patients in whom dysplasia was classified as ‘not otherwise specified’ had conversion to malignancy within three months. Consequently, this severity of dysplasia was not analysed any further in respect to malignant transformation cases. Of the 32 patients in whom malignant transformation occurred at any stage, 13 patients (40.1 per cent) had transformation within 12 months and 23 patients (71.9 per cent) within 60 months. Excluding those who had rapid progression to carcinoma, occurring within 12 months, 19 of the remaining 112 patients with initial dysplasia (16.9 per cent) had conversion to invasive malignancy within 10 years.
In this study, mild, moderate and severe dysplasia appeared to behave as three separate entities with respect to malignant transformation rate. Particularly, we identified that moderate dysplasia had a considerable malignant transformation rate, closer to severe than mild. When the moderate and severe dysplasia groups were combined into a ‘high-grade dysplasia’ group, conversion to malignancy represented 85 per cent (22 out of 26) of all malignant transformations. The remaining four ‘low-grade lesions’ represent all those specimens reported as mild dysplasia. The malignant transformation rate for high-grade lesions was found to be 27.5 per cent over 10 years, compared with 10.8 per cent for low-grade lesions. The Pearson chi-square test revealed a significant difference (p = 0.043) in the rate of malignant transformation when low and high grades of dysplasia were classified in this way.
When the time to malignant transformation was plotted on a Kaplan–Meier survival curve, a log rank test demonstrated that the mean length of time (in months) was statistically different when comparing mild, moderate and severe dysplasia (p = 0.037) (Figure 3), as well as high- versus low-grade dysplasia (p = 0.012) (Figure 4). Additionally, there was a statistically significant difference between the mean length of time to malignant transformation for the three grades of dysplasia, mild, moderate and severe, as determined by one-way analysis of variance (64 ± 21.2 months, 46.4 ± 37.1 months and 10.3 ± 4.8 months, respectively; p-value = 0.031). A Tukey post-hoc test revealed that the time to malignant transformation was statistically significantly lower in patients with severe dysplasia (10.3 ± 4.8 months; p = 0.048) compared with patients with moderate dysplasia (46.4 ± 37.1 months).

Fig. 3. Kaplan–Meier survival curve demonstrating time to malignant transformation of mild, moderate and severe dysplasia cases.

Fig. 4. Kaplan–Meier survival curve demonstrating time to malignant transformation of low- and high-grade dysplasia cases.
The 10-year all-cause mortality rate was higher for the malignant transformation group compared with the non-transformation group (46 per cent vs 15 per cent). Salvage total laryngectomy was performed in 30 per cent of malignant transformation cases. Important clinical outcome measures, including mortality rates and salvage laryngectomy rates, are described in Table 3.
Table 3. Demographics and secondary outcome measures for all malignant transformation cases

Data on the T stage of the subsequent developing cancer are shown in Figure 5, demonstrating that 58 per cent of subsequently diagnosed cancers were stage 1. The timescale of malignant transformation was highly variable for each stage, with no discernible relationship. The mean time to diagnosis of SCC for each T stage, T1 to T4, was 43 months, 29 months, 102 months and 33 months, respectively.

Fig. 5. Squamous cell carcinoma (SCC) stage at time of diagnosis in patients with prior dysplasia of any severity.
The details of the treatment modality are displayed in Table 4. Radiotherapy was utilised in two cases: one because of difficult access to the anterior commissure with laser and the other as the outcome of multidisciplinary team discussion.
Table 4. Treatment modality utilised following initial histopathological dysplasia diagnosis

NOS = not otherwise specified
In an attempt to aid our understanding of risk factors for progression to invasive carcinoma, we noted the treatment modality used in each grade of dysplasia that subsequently converted. These data are displayed in Table 5. The Fisher's exact test indicated no significant difference between the treatment type and the chance of malignant transformation (p = 0.171).
Table 5. Treatment modality used in dysplasia cases that subsequently developed into invasive cancer

Discussion
This regional retrospective cohort study is one of the largest studies of laryngeal dysplasia in the UK, including 119 patients with a histopathological diagnosis of dysplasia affecting the glottis. An important observation identified during data collection was the high rate of synchronous head and neck cancers. Eleven patients were diagnosed with a synchronous tumour, and seven had evidence of a previous head and neck cancer or local radiotherapy. This should highlight the spectrum of mucosal changes that can occur along the entire upper aerodigestive tract.
Our results agree with other major studies and cancer incidence data indicating that laryngeal cancer predominantly affects male patients.Reference Donnelly and Gavin19,20 There is a widely reported malignant transformation rate of laryngeal dysplasia ranging between 2 and 74 per cent. Our results show an overall malignant transformation rate of 21.8 per cent, and demonstrated a higher risk and shorter mean time to transformation with increasing dysplasia severity. This would seem to disagree with the finding of a meta-analysis by Weller et al. in 2010, which concluded that the transformation time was not dependent on the grade of dysplasia.Reference Weller, Nankivell, McConkey, Paleri and Mehanna1 When considering each grade of dysplasia, our results showed that mild dysplasia was associated with a low rate of progression to carcinoma of 10.8 per cent, very similar to the value of 10.6 per cent reported by Weller et al.Reference Weller, Nankivell, McConkey, Paleri and Mehanna1 The same meta-analysis combined moderate and severe lesions, producing a transformation rate of 30.4 per cent, similar to 27.5 per cent as shown in this study. A similarly sized study in Paisley, Scotland showed a transformation rate of 16 per cent, with a mean time to diagnosis of 43 months.Reference Montgomery and White3 A further study based in Newcastle with 113 patients demonstrated a transformation rate of 24 per cent, with 75 per cent occurring within 12 months.Reference Jeannon, Soames, Aston, Stafford and Wilson21 A very recent publication from Denmark showed a transformation rate of 18.3 per cent among 965 patients with laryngeal dysplasia.
When considering all 125 cases of dysplasia, 40 per cent of patients proceeded to have invasive carcinoma diagnosed on subsequent biopsies within 12 months and 72 per cent within 60 months. This high number of patients within a ‘rapid progression to carcinoma’ group may represent those with very unstable mucosal changes at high risk of subsequent invasion, or is perhaps a consequence of tissue sampling errors. Our study design excluded those patients in whom SCC was diagnosed within three months of their primary biopsy. The rationale for this was two-fold: firstly, this was a standard adopted by many of the other major studies in this area and thus would allow comparison of results; and secondly, the diagnosis of dysplasia could represent a tissue sampling error. Consequently, clinicians should carefully follow up all patients with dysplasia, particularly within the first year, with a low threshold for repeating biopsy or instigating appropriate treatment.
An interesting finding within the biopsy samples of all 76 cases of SCC is that co-existing epithelial dysplasia was reported for at least 74 per cent of samples, with the reports for the remaining samples not explicitly recording this. This is not insignificant, considering that a tissue sampling error could have resulted in a biopsy that missed the invasive cancer and only captured an area of dysplasia. A large meta-analysis also concluded that laryngoscopy is inadequate for the detection of dysplastic and malignant lesions, in that only 47.1 per cent of clinical leukoplakia revealed dysplasia.Reference Isenberg, Crozier and Dailey22 These findings reaffirm the concerns of reliance on histopathological reports.
All grades of dysplasia – mild, moderate and severe – appeared to behave as separate entities, with increasing rates of progression to carcinoma noted as dysplasia severity increased. Moderate dysplasia was the most commonly identified degree of dysplasia, representing 46 per cent of all biopsies. This finding may reflect our arbitrary application of a distinct classification system to a spectrum of mucosal changes, meaning that most biopsies will contain changes that lie somewhere in the middle. There has been much debate about the malignant potential of moderate dysplasia. Our results would tend to agree with Luers et al.,Reference Luers, Sircar, Drebber and Beutner23 who concluded in 2014 that moderate dysplasia confers a significant conversion risk. In our study, 24.0 per cent of moderate dysplasia cases progressed to invasive cancer, which clearly represents a significant risk.
When moderate and severe dysplasia groups were combined into a ‘high-risk’ group, this constituted 85 per cent of all patients who subsequently developed SCC. Combining these two groups would certainly identify the majority of lesions at higher risk of SCC on future biopsies. If this more heterogeneous group were recognised, it might harmonise these high-risk lesions, so as to minimise the worry of over-treating and to negate the concern of inter-observer variability in reporting. Combining the classification system of dysplasia from four tiers to two, and in certain circumstances three, was the recommendation of the WHO in 2017.Reference Gale, Poljak and Zidar24 Our results would appear to support that decision. Our findings would also support the use of more aggressive treatment of patients with severe dysplasia, as 34.6 per cent had subsequent evidence of invasive carcinoma within a mean time frame of 28 months.
Our results showed a statistical difference in the mean time to malignant transformation for both the two-tier and the three-tier classification system. The mean time for high-grade lesions was 43 months, compared with 101 months for low-grade lesions. Although there were only four patients within the low and mild dysplasia groups, the earliest point at which one of these patients had further evidence of SCC was after four years. High-grade lesions seem to confer a higher risk within a shorter time period. The SCC was diagnosed between 4 and 132 months after the index biopsy showing dysplasia. This clear variability highlights the difficulty for clinicians in planning the duration of surveillance.
There was no apparent association between the initial grade of dysplasia and the T stage of the subsequently diagnosed SCC. This would support the general principle that dysplasia in itself is a precursor to malignancy, but not in a reproducible pattern.
Our study demonstrates the preferential use of CO2 laser over other modalities for managing high-grade dysplasia. Although this study showed a tendency to use laser therapy, there was no significant difference between treatment modalities in terms of the risk of malignant transformation. The wide variation of treatment protocols limits direct comparison between studies.
• Laryngeal dysplasia confers a considerable malignant potential
• The malignant transformation rate rises with increasing dysplasia severity
• Each degree of dysplasia severity appears to behave as a separate entity, with moderate dysplasia conferring a higher potential malignant risk than previously described
• By classifying dysplasia into a two-tier classification system of high- and low-grade dysplasia, prognostic information can be improved, but this risks including more heterogeneous patient groups
• Duration and frequency of surveillance should consider dysplasia severity in addition to recognised patient and disease risk factors
Our results showed that mild dysplasia tends to progress to invasive carcinoma over a long period of time (mean of 101 months), which may not be served well by rigorous invasive monitoring in the short term. However, the possibility of tissue sampling errors always merits consideration. Our results show a significant malignant potential of moderate dysplasia (24.0 per cent), a risk that should not be considered inconsequential. Ultimately, we cannot lose sight of the clinical aspects of this disease. There is currently insufficient evidence to suggest that symptom recurrence correlates with presentation at an early stage; therefore, there appears to be a lack of evidence to advocate the early discharge of patients with low-grade dysplasia.
Conclusion
Laryngeal dysplasia carries a significant risk of malignant transformation. This risk appears to increase with dysplasia severity. This study advocates the combination of moderate and severe dysplasia into a high-grade group. In this study, the high-grade group demonstrated a higher malignant transformation rate (27.5 per cent vs 10.8 per cent; p = 0.043) and shorter mean time to transformation (43 months vs 101 months; p = 0.012), when compared with low-grade or mild dysplasia. There is a high rate of malignant progression within the first 12 months of initial diagnosis, but transformation can still occur late. Mild, moderate and severe dysplasia appear to behave as separate entities, with a statistical difference identified in their mean time to transformation (101 months, 52 months and 28 months, respectively; p = 0.037). This study does not conclude that one particular treatment modality confers a higher cure rate or survival rate for laryngeal dysplasia.
Laryngeal dysplasia, specifically affecting the glottis, is a multifactorial process, influenced by a plethora of confounding factors, some of which, such as smoking and male gender, have been demonstrated within this study. A diagnosis of laryngeal dysplasia carries a high morbidity and mortality rate. There is a well-recognised rate of tissue sampling error and inter-observer variability that should lead to caution when interpreting a histopathological report without sufficient clinical information. We must highlight the crucial role of the multidisciplinary team approach and allow for patient autonomy when deciding upon the appropriate treatment and surveillance of this complex patient group.
Competing interests
None declared












