Introduction
Herpes simplex virus is a ubiquitous virus but infection is uncommon in the larynx of immunocompetent patients. The site of herpes simplex virus infection is most commonly the upper aerodigestive tract or genitalia, depending on subtype. Herpes simplex virus 1 is conventionally associated with non-sexual contact and presents as gingivostomatitis or pharyngitis, while herpes simplex virus 2 usually presents as vesicular lesions in the anogenital areas. However, herpes simplex virus 1 has recently emerged as an important cause of genital herpes in certain developed countries, and an increasing proportion of oral infections are being attributed to herpes simplex virus 2.Reference Xu, Sternberg, Kottiri, McQuillan, Lee and Nahmias1, Reference Eveson, Cardesa and Slootweg2 Regardless of subtype, herpes simplex virus infections tend to be subclinical and up to one-third of patients are susceptible to recurrent infection.Reference Eveson, Cardesa and Slootweg2 Atypical or severe presentations of herpes simplex virus infection are more common in immunosuppressed patients.
Case report
An 82-year-old woman was referred by her general practitioner to the ENT department of Charing Cross Hospital, London, in May 2014 with a 4-month history of dysphonia. She did not have shortness of breath, and denied any difficulty or pain in swallowing. There were no systemic symptoms of weight loss, malaise or fever.
Her medical history included rheumatoid arthritis, for which she had been treated with subcutaneous etanercept (a tumour necrosis factor inhibitor) injections until November 2013, when the treatment was switched to once-weekly 17.5 mg methotrexate. Other co-morbidities included left ventricular failure, atrial fibrillation, hypertension and mild, well-controlled asthma. She had a 20 pack-year history of smoking and did not drink alcohol. Two months after she started methotrexate treatment, she developed progressive dysphonia.
Fibre-optic nasoendoscopy showed friable ulcerated lesions affecting both vocal folds. Her airway was otherwise not compromised and no other mucocutaneous lesions were present. Owing to a strong suspicion of malignancy, she underwent urgent microlaryngoscopy; this revealed erythematous vocal folds and small shallow ulcers affecting the anterior two-thirds of her vocal folds (Figure 1). Biopsies were taken for histological examination.
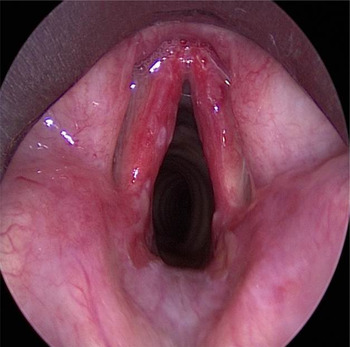
Fig. 1 Operative photograph taken with a 0° rigid endoscopy, showing bilateral erythema and vocal fold ulceration at presentation.
Biopsies from the mid-left vocal fold showed surface ulceration with reactive atypia but no definite features of viral inclusions. Biopsies from the right anterior vocal fold showed ulcerative squamous epithelium with viral cytopathic changes indicative of infection with herpes simplex virus, which was confirmed as herpes simplex virus 1 on immunohistochemical analysis (Figures 2 and 3). Fungal staining was negative. There was no evidence of dysplasia or malignancy at either biopsy site.
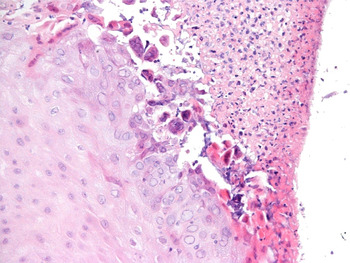
Fig. 2 Photomicrograph of the biopsy site showing ulceration of the squamous epithelium and viral inclusions characteristic of herpes simplex virus infection (H&E; ×200).

Fig. 3 Photomicrograph showing positive immunohistochemical staining for herpes simplex virus 1 in multinucleate cells (×400).
Given the extent of the clinical presentation, it was considered appropriate to treat the herpes simplex virus infection with oral antivirals with a view to withdrawing methotrexate if there was no response. The patient was promptly started on a two-week course of 200 mg oral acyclovir five times a day, and a clinical decision was made not to discontinue methotrexate treatment in the interim. At the end of the treatment period, rigid strobolaryngoscopy showed complete resolution of the herpetic lesion, and a normal appearance for both vocal folds (Figure 4). Clinically, the patient remained systemically well with no further complaints of dysphonia or dysphagia. A computed tomography scan of her neck confirmed symmetrical vocal folds with no evidence of a mucosal abnormality within the upper aerodigestive tract and no pathological lymphadenopathy in the neck. Interestingly, despite no alterations being made to the methotrexate dose throughout the treatment period, there has been no recurrence of herpes simplex virus infection.
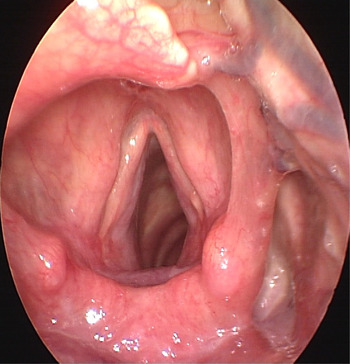
Fig. 4 Post-treatment photograph taken with a 70° rigid strobolaryngoscope showing complete resolution of the lesions.
Viral culture or serological tests did not form part of the pre-therapy assessment, and were not performed following complete clinical resolution because they would have been uninformative.
Discussion
A handful of case reports of herpes simplex virus laryngitis have been published. O'Niel et al. reviewed the number of immunocompetent children (excluding neonates) with confirmed herpes simplex virus laryngitis: of the children presenting with atypical features of croup or laryngitis, 50–70 per cent were retrospectively found to have ulcerative stomatitis coinciding with laryngitis either at presentation or during the course of their protracted illness.Reference O'Niel, Chun and Conley3 In the context of an atypical presentation of croup or localised ulceration, these non-specific symptoms may guide the clinician towards further investigation of possible herpes simplex virus infection and the prompt administration of empirical treatment.Reference O'Niel, Chun and Conley3
In the adult population, diagnosing herpes simplex virus laryngitis can be challenging, particularly if a temporal relationship with virus exposure is not known or suspected. Generally, the symptoms of herpes simplex virus infection in the larynx are identical to those of any infective or inflammatory process in the mucosa. These include the presence of white plaques resembling leukoplakia, mucosal ulceration (as in this patient) or even a large discrete mass.Reference Sims, Massoll and Suen4 Therefore, the most important differential diagnosis is a primary laryngeal malignancy. Herpes simplex virus laryngitis is rarely considered a competing differential diagnosis until tissue is submitted for histological examination or unless there is already a strong clinical suspicion. The typical cytological features of herpes are intercellular oedema with keratinocyte ballooning and vacuolisation, resulting in vesiculation.Reference Eveson, Cardesa and Slootweg2 Nuclei are enlarged, with nuclear inclusions and a clear halo (Lipschutz bodies or Cowdry type A inclusions), along with nuclear moulding and ‘ground-glass’ chromatin; multinucleated epithelial giant cells are associated with extensive inflammation and there is progressive ulceration.Reference Eveson, Cardesa and Slootweg2
There are also case reports of herpes simplex virus infection in the larynx associated with bilateral vocal fold abductor paralysis (Gerhardt syndrome) that rapidly resolved following antiviral therapy.Reference Dupuch, Saroul, Aumeran, Pastourel, Mom and Gilain5 Despite the lack of definitive evidence for herpes simplex virus as a primary aetiological agent in laryngeal palsy, a combination of clinical history, serology findings and therapeutic improvement supports this unique and rare entity.Reference Dupuch, Saroul, Aumeran, Pastourel, Mom and Gilain5
• Herpes simplex virus infection is common in immunosuppressed patients
• Diagnosing a laryngeal infection can be challenging
• The most important differential diagnosis is a primary laryngeal malignancy
• Isolated infection in the larynx is an unusual and rare complication of methotrexate therapy
The most common cause of herpes simplex virus infection at an atypical site is immunosuppression or immunodeficiency. Herpes simplex virus laryngitis associated with acquired immunodeficiency syndrome was first reported in 1994 in a patient presenting with the clinical features of chronic hoarseness and leukoplakia on direct laryngoscopy, which required histopathological and immunohistochemical analysis for diagnosis.Reference Yeh, Hopp, Goldstein and Meyer6 Aciclovir-resistant oral lesions have been reported in the context of bone marrow transplantation and immunosuppression.Reference Brooke, Eveson, Luker and Oakhill7
In the context of inflammatory arthropathy treatment, methotrexate is well known to have immunosuppressive properties. Patients may develop oral and gastrointestinal mucositis and ulceration. Other recently reported methotrexate-associated oral lesions in rheumatoid arthritis patients include lymphoproliferative disorders of the tongue and Epstein-Barr virus associated mucocutaneous ulcers, which clinically regressed following withdrawal of methotrexate.Reference Hashimoto, Nagao, Saito and Kinoshita8, Reference Sadasivam, Johnson and Owen9
There are case reports of severe herpes simplex virus infection involving a wide variety of anatomical sites, including the lung, liver, eye, central nervous system and skin following treatment with methotrexate and other biologic agents.Reference O'Connor and Phelan10 However, to date there have been no published reports of herpes simplex virus infection localised to the larynx in the background of methotrexate-induced immunosuppression.
Conclusion
To our knowledge, this is the first report of a rare complication of herpes simplex virus infection in the context of methotrexate-induced immunosuppression. In extreme cases, this complication may present a therapeutic challenge for conditions which rely on the immunosuppressive effects of methotrexate and other similar agents.






