Introduction
The petrous apex is a pyramidal-shaped region of the petrous temporal bone, with its base medial to the otic capsule and its apex at the clivus. Anteriorly, lies the greater wing of the sphenoid, and anterolaterally the carotid canal, tensor tympani muscle and Eustachian tube. Posteriorly, lies the internal auditory meatus and occipital bone with which it forms the jugular foramen. Superiorly within the apex is a depression for the trigeminal ganglion.
The relevance of a detailed appreciation of the anatomy and pneumatisation of the temporal bone becomes apparent when comprehensively removing all mucus-secreting mucosa from cellular tracts, and obliterating the middle ear and Eustachian tube (termed subtotal petrosectomy and blind sac closure). This may be necessary as part of tumour removal, for recalcitrant chronic otitis media or to repair cerebrospinal fluid (CSF) leaks. The success of this procedure is dependent upon the removal of all air cells, and the structural separation of the intracranial space and middle ear or mastoid from the nasopharynx and ear canal.
In the temporal bone, the Eustachian tube provides ventilation to the middle-ear and mastoid air cells. The degree of temporal bone pneumatisation varies significantly from person to person, and although relatively symmetrical in most cases,Reference Lindsay1 it can vary from one side to the other. The degree of pneumatisation is widely thought to be dependent on Eustachian tube function, with poor ventilation contributing to chronic middle-ear inflammation and mastoid sclerosis.Reference Gleeson2 In a well-pneumatised petrous bone, air cells can be present around the posterior opening of the Eustachian tube (peri-tubal); they can be found in relation to the internal carotid artery (peri-carotid); they can exist above and below the otic capsule (supralabyrinthine and infracochlear regions respectively); and there can be pneumatisation of the petrous apex itself.
Of note, peri-Eustachian tube cell pneumatisation is known to be highly variable and can be quite extensive (Figure 1).Reference Lindsay1–Reference Jen, Sanelli, Banthia, Victor and Selesnick4 Saim et al. found 65 per cent of their temporal bones on histological analysis had peri-tubal pneumatisation, with 91 per cent of the cells opening into the Eustachian tube (several millimetres) anterior to its tympanic orifice, and 9 per cent opening into the middle ear.Reference Saim, McKenna and Nadol5 Lindsay's anatomical and radiological studies of temporal bones, published in 1940, determined multiple pneumatisation tracts presumed to be spreading from the mastoid, middle-ear or peri-tubal cells to the apex.Reference Lindsay1 These involved the infralabyrinthine (inferior to the otic capsule), posteromedial (along the posterior fossa dura then anterior to the internal auditory canal), subarcuate (following the subarcuate artery), anterior (between the cochlea and the internal carotid artery) and superior (supralabyrinthine) regions.Reference Lindsay1
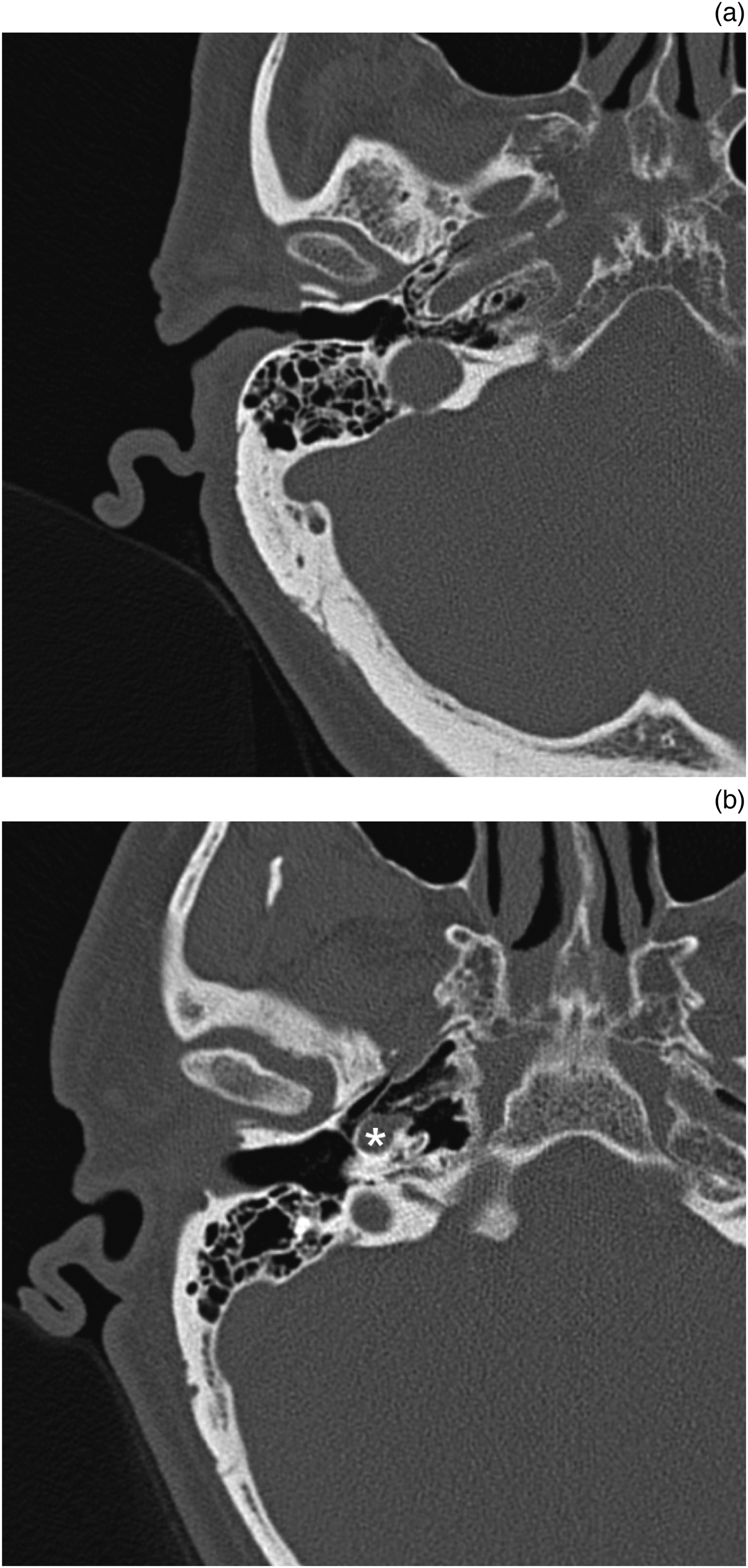
Fig. 1. Examples of well-pneumatised petrous temporal bones on axial computed tomography. (a) Typical configuration with peri-carotid and peri-tubal pneumatisation. (b) Demonstrates an internal carotid artery (asterisk) with a mesentery separating a highly pneumatised peri-tubal cell system and petrous apex from the middle ear.
These tracts play a part in the development of petrous apex pneumatisation, but also provide potential pathways for the spread of infection and other disease processes. Although petrous apex pneumatisation has been hypothesised to begin from the middle-ear, mastoid or peri-tubal cells,Reference Lindsay6, Reference Roland, Meyerhoff, Judge and Mickey7 this has not been demonstrated conclusively, and the age at which this occurs is not known. Apex pneumatisation is said to occur in 11–35 per cent of individuals,Reference Lindsay1, Reference Virapongse, Sarwar, Bhimani, Sasaki and Shapiro3, Reference Grant, Welling, Oehler and Baujan8–Reference Hindi, Alazzawi, Raman, Prepageran and Rahmat10 and generally arises in a symmetric fashion in the absence of chronic suppuration.Reference Lindsay1, Reference Hindi, Alazzawi, Raman, Prepageran and Rahmat10 Five per cent to 10 per cent of cases display an asymmetric marrow pattern.Reference Virapongse, Sarwar, Bhimani, Sasaki and Shapiro3, Reference Roland, Meyerhoff, Judge and Mickey7, Reference McRackan and Brackman11
The authors hypothesise that in most patients the carotid artery separates cellular tracts posteriorly from those anteriorly, with no second communication medial to it. The clinical implication of this being that if all air cells (posteriorly) up to and including those overlying and lateral to the vertical intratemporal carotid are exenterated, the long-term success rate of subtotal petrosectomy and blind sac surgery should be improved. If the carotid is an effective landmark barrier (i.e. the only communication path being lateral to the artery) separating cellular tracts behind from those in front, with no second communication medial to it, then regarding the carotid as the medial limit of dissection in a subtotal petrosectomy would theoretically ensure complete separation of spaces. This would increase the chance of a successful surgical outcome, and reduce the risks of persistent CSF leak and enduring disease (Figure 2).Reference Jen, Sanelli, Banthia, Victor and Selesnick4

Fig. 2. Intra-operative photograph following completion of right subtotal petrosectomy (note: all visible air cells have been exenterated). ‘a’ = facial nerve; ‘b’ = cochlear promontory; ‘c’ = vertical segment internal carotid artery; ‘d’ = Eustachian tube orifice
This study aimed to ascertain in what proportion of patients the carotid artery represented the medial border separating the middle ear from the nasopharynx.
Materials and methods
Both sides of 222 consecutive high-resolution (0.75 mm slice thickness) computed tomography (CT) scans of petrous temporal bones, performed for various otological and neurotological indications, at a single tertiary referral centre, from January 2014 to September 2016, were retrospectively reviewed using an Agfa Healthcare ICIS™ View (version 2014.1.SU7.14) medical images and results viewer.
The scans were reviewed to ascertain whether the carotid artery represented a cellular separation from the Eustachian tube (and its associated peri-tubal cells), and to assess for pneumatisation of the petrous apex. It was also noted whether a pneumatised petrous apex had air cells that were radiologically separate from the middle-ear cells or whether the two were connected via a tract. All images were viewed in the bone window setting, and both coronal and sagittal planes were examined.
Although there is no evidence to suggest at what age pneumatisation of the petrous apex is complete, patients younger than 16 years old were excluded from the main study in an attempt to standardise the degree of pneumatisation in the scans reviewed.
The sclerosed mastoids of the 90 chronic otitis media patients were also analysed as a separate subgroup, as pneumatisation is typically reduced in these patients.Reference Gleeson2 In addition, both sides of 29 consecutive paediatric (0–16 years old) CT scans were reviewed, to establish evidence of the process of petrous apex pneumatisation.
Results
In the adult group, the patient age ranged from 16 to 88 years (mean, 52 years). The percentage of CT scans in which the carotid artery on its medial aspect separated cellular tracts posteriorly from peri-tubal/Eustachian tube cells anteriorly was 96.4 per cent for the right side and 95 per cent for the left (the average for both sides was 95.7 per cent). Excluding the 90 patients clinically deemed to have chronic otitis media, the right side and left side values equated to 93.9 per cent and 93.2 per cent respectively (total average of 93.6 per cent).
In the adult study population, the petrous apex was pneumatised in 5.4 per cent of patients on the right side, and in 7.7 per cent on the left side. When the petrous apex was pneumatised, 86.2 per cent of these were visibly connected to the middle-ear air cell system; in the remaining 14 per cent, they were not.
Within the paediatric group, the petrous apex was pneumatised in only 1 out of 29 patients (3 per cent), with a further 3 out of 29 patients (10 per cent) demonstrating visible air cell tracts (such as the infracochlear and/or supralabyrinthine tracts) approaching, but not reaching, the petrous apex (Figure 3). In the single case with a pneumatised petrous apex, the patient had undergone CT scanning, at 5 and 11 years for sensorineural hearing loss, and subsequent post-cochlear implant insertion review. The images demonstrated progressive petrous apex pneumatisation, spreading from a superior to inferior direction, originating from the middle-ear or mastoid air cells (Figure 4).

Fig. 3. Computed tomography scans showing (a) Supralabyrinthine (7 years old) and (b) infracochlear (14 years old) paediatric air cell tracts. (c) Extensive peri-tubal pneumatisation in a five year old. (a, b) Coronal plane and (c) axial plane.

Fig. 4. Computed tomography scans showing progressive paediatric petrous apex pneumatisation. Images (a) and (b) show same patient at 5 and 11 years old respectively. Both images are in the coronal plane.
The earliest signs of apex pneumatisation were found in a five year old. Peri-tubal pneumatisation was present in 3 out of 29 paediatric patients (10 per cent), and was also observed to occur from five years of age.
Discussion
This study has shown that in 96 per cent of temporal bones, the carotid artery can be regarded as a landmark dissection barrier separating the Eustachian tube from the cellular spaces posteriorly, as there is no secondary cellular tract on its deep aspect. Therefore, if the spaces posteriorly up to and lateral to the vertical intratemporal carotid are definitively obstructed, then there is a 96 per cent chance that this alone will succeed in separating the middle ear from the nasopharynx. In the remaining 4 per cent in whom a connection deep to the carotid exists, obstruction laterally in isolation may well result in a persistent CSF leak, or residual or recurrent chronic otitis media symptoms.
• Temporal bone pneumatisation is variable; pneumatisation tracts can be multiple, providing potential pathways for infection and disease
• Subtotal petrosectomy and blind sac closure success depends on air cell removal, and separation of intracranial space and middle ear/mastoid from nasopharynx and ear canal
• In adults, petrous apex was pneumatised in 6.5 per cent, and 86 per cent of these communicated with middle-ear air cell system
• In children, petrous apex pneumatisation occurred in 3 per cent
• In 96 per cent of temporal bones, carotid artery forms a lateral barrier between air spaces anterior and posterior
• Regarding the carotid as the medial limit of dissection in subtotal petrosectomy and blind sac closure should optimise surgical outcome
Adequate management of this small percentage requires blockage of this alternative path, which cannot be accessed from behind because of the limiting proximity of the cochlea and jugular bulb. This therefore necessitates an approach skirting forwards of the carotid and then deeply, to occlude these forwards-intruding apex cells. To ensure certainty of this approach requires a similar full length exposure of the vertical carotid artery. Regarding the vertical intratemporal carotid artery routinely as the perimeter limit of dissection during a subtotal petrosectomy should therefore confer the greatest chance of surgical success, and the lowest likelihood of recurrence or failure.
Unsurprisingly, the chronic otitis media cases, with their sclerosed mastoids and middle-ear air cell systems, were more likely to have this front-to-back separation deep to the carotid.
This study found a lower rate of petrous apex pneumatisation (6.6 per cent) compared with published series (11–35 per cent), presumably secondary to the high rate of Eustachian tube dysfunction and chronic otitis media found in our population, which most studies excluded.Reference Lindsay1, Reference Virapongse, Sarwar, Bhimani, Sasaki and Shapiro3, Reference Jen, Sanelli, Banthia, Victor and Selesnick4, Reference Lindsay6, Reference Grant, Welling, Oehler and Baujan8–Reference Hindi, Alazzawi, Raman, Prepageran and Rahmat10 However, our study is potentially the largest performed to date that assesses apex pneumatisation, and hence is significant in eliciting the true pneumatisation incidence in a real-world scenario, wherein imaging is often acquired in the presence of chronic disease. In contrast to many of the previous studies from the 1980s and 1990s, the resolution of CT scanning has markedly improved, so our findings are likely to be more accurate. Indeed, Virapongse et al. suspected that the thick-section CT scans used in their analysis may have been subject to considerable partial volume effects, accounting for their ‘spurious findings’.Reference Virapongse, Sarwar, Bhimani, Sasaki and Shapiro3
An aerated petrous apex was more likely to be found with widespread aeration of the associated air cells tracts and mastoid, in keeping with good Eustachian tube function. If the petrous apex is aerated, then it follows that 86 per cent of these have clear connections to the middle-ear ventilation system. The remainder may also have connections to the middle ear, but these were not visible radiologically. One of Lindsay's key findings was that petrous infections almost exclusively occurred in pneumatised bones, with the infection originating in the middle ear and spreading along air cell tracts to the apex.Reference Lindsay1
Only 3 per cent of the paediatric population demonstrated petrous apex pneumatisation. Our serial CT scans provide evidence for the process of aeration originating from the middle ear and invaginating into the apex, similar to maxillary sinus development.Reference Gleeson2 This can be visible in those as young as five years, though it potentially exists at an earlier age. Anson et al. reported a case of petrous temporal bone pneumatisation in a child aged four and a half years, which appears to be the earliest that this has been noted to occur in the literature.Reference Anson, Wilson and Gaardsmoe12
Limitations of the study include the reliance on a solely radiological analysis, without subsequent histopathological or clinical correlation. However, given the high resolution of the CT scans reviewed, we believe the addition of these data would not significantly alter the results. Nonetheless, there is a limit to the resolution of CT scanning, which is subject to partial volume averaging effects. Whether these radiological findings relate accurately to clinical outcomes is debatable. However, Lyndsay's earlier study provides direct clinical and histological correlation between radiologically visible tracts and associated pathology, suggesting such evident air cell tracts to be patent enough to: be clinically significant, permit aeration of the petrous apex and contribute to the spread of disease.Reference Lindsay1, Reference Lindsay6 The interpretation of our scans was open to a degree of subjectivity. In addition, although the paediatric sample size was small, severely limiting our ability to infer conclusions regarding the pneumatisation process, it is still one of the largest to date.
The clinical implications of this study include the need to attend to the carotid artery as well as obliteration of the Eustachian tube, in order to optimise surgical outcomes during subtotal petrosectomy and blind sac closure. Compared with a well-aerated bone, in chronic otitis media the incidence of petrous apex disease is likely to be lower, and the probability of subtotal petrosectomy being successful with Eustachian tube obliteration alone (without attention to the carotid area) is perhaps slightly higher. Having said that, there is still a risk of operative failure if the carotid is not adequately delineated; this applies to both normally aerated and chronic otitis media (sclerosed) temporal bones.
Competing interests
None declared






