Introduction
Hypoxia induces changes that vary depending on its duration and the type of body tissue affected.Reference Rothman and Olney1 Low levels of oxygen in the blood induce a shift in cellular respiration from aerobic (mitochondrial oxygenation) to anaerobic respiration, which eventually causes a build-up of lactic acid.Reference Puschner and Schacht2 In early life, exposure to critical levels of hypoxia leads to birth asphyxia and hypoxic ischaemic encephalopathy.Reference Lipton3
The effect of postnatal hypoxia on the hearing organ has been studied in vitro by assessing cultured outer hair cells' survival in a hypoxic environment.Reference Mazurek, Winter, Fuchs, Haupt and Gross4 Similarly, hypoxia has been modelled in adult chinchillas; in that particular study, the hearing organ was assessed using cochlear action potentials and scanning electron microscopy.Reference Shirane and Harrison5
Clinical studies looking at the hearing outcome of asphyxiated children at six months and one year of age have reported significant hearing loss using both otoacoustic emissions and auditory brainstem response (ABR) testing.Reference Ide, Harada, Kanzaki, Saito, Hoshikawa and Kawahara6–Reference Jiang, Yin, Shao and Wilkinson8 However, other studies have indicated that hearing loss which occurs as a result of hypoxia is not permanent as it is reversed when normoxia resumes.Reference Sohmer, Gafni and Chisin9, Reference Attias, Sohmer, Gold, Haran and Shahar10
In this study on Sprague–Dawley rats, we investigated the impact of experimentally induced postnatal hypoxia on ABR thresholds, wave I latencies and wave I–V inter-peak latencies, in adulthood. Our aim was to determine the long-term effect on hearing in this group of rats exposed to 10 days of neonatal hypoxia followed by normoxia.
Materials and methods
Pregnant Sprague–Dawley rats were purchased at 17–18 days gestation (from Charles River, Montreal, Canada).
After birth, each litter was culled to all male pups. Only male pups were used in order to avoid gender- or hormone-related differential effects of hypoxia that could potentially bias the results.
Half of the litters were reared in normoxia, while the other half were reared in hypoxia (fraction of inspired oxygen (FiO2) = 0.12) from days 1 to 10 of life, and subsequently reared in normoxia. A total of 22 male pups (10 controls and 12 hypoxic) were utilised for this study. The animals had food and water ad libitum and were regularly monitored for activity levels and body weights. The study received approval from the Institutional Review Board of the Research Institute of McGill University Health Centre.
Mode of hypoxia exposure
On the first day of life, rat pups in the experimental group were placed with their mother in a chamber that provided a constant flow of hypoxic air (FiO2 = 0.12). This was achieved by mixing nitrogen with air, verified by an oxygen analyser.
Hearing assessment
Each animal had its hearing assessed in adulthood (at approximately 72 days old) using ABR testing (IHS Technologies, Miami, Florida, USA). Hearing thresholds were determined at 4, 8, 16, 25 and 32 kHz.
The active electrode was inserted in the scalp, between the ears and over the vertex of the skull. A reference electrode was placed subcutaneously in the auricle of the tested ear, and a ground electrode was placed in the contralateral ear. Sound was delivered using a closed system, with probes carefully nestled into the ear canal. Brief tone stimuli were presented at 1600 sweeps per second for 31.3 ms, at a rate of 39.1 per second. The test was performed at five different frequencies (4, 8, 16, 25 and 32 kHz), and the threshold, which was the minimum intensity at which a certain frequency could be processed, was obtained manually for both ears.
Data analysis
The ABR thresholds and wave latencies obtained for each group are reported as means. Statistical significance was established using the paired t-test (GraphPad software, La Jolla, California, USA), drawing significance of data from two-tailed p values and scientific significance from 95 per cent confidence intervals.
Results
The control animals were euthanised at 72.8 ± 2.7 days, while the mean age of the hypoxic rats at euthanasia was 71 ± 2.8 days. The weights of the control animals were significantly higher than those of the hypoxic rats. The mean weights of control and hypoxic rats were 501 ± 41.8 g and 363 ± 37 g, respectively (p < 0.05).
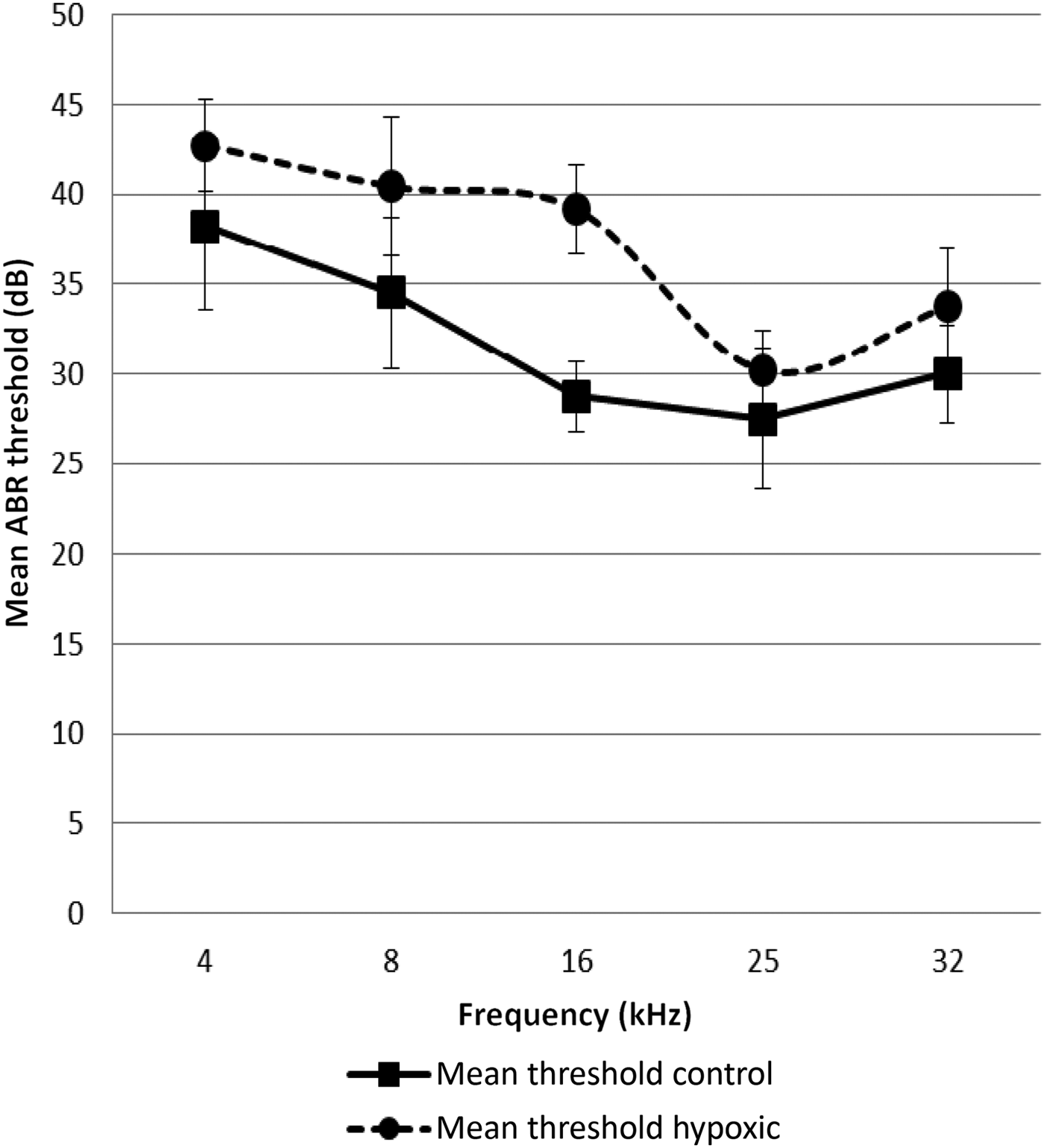
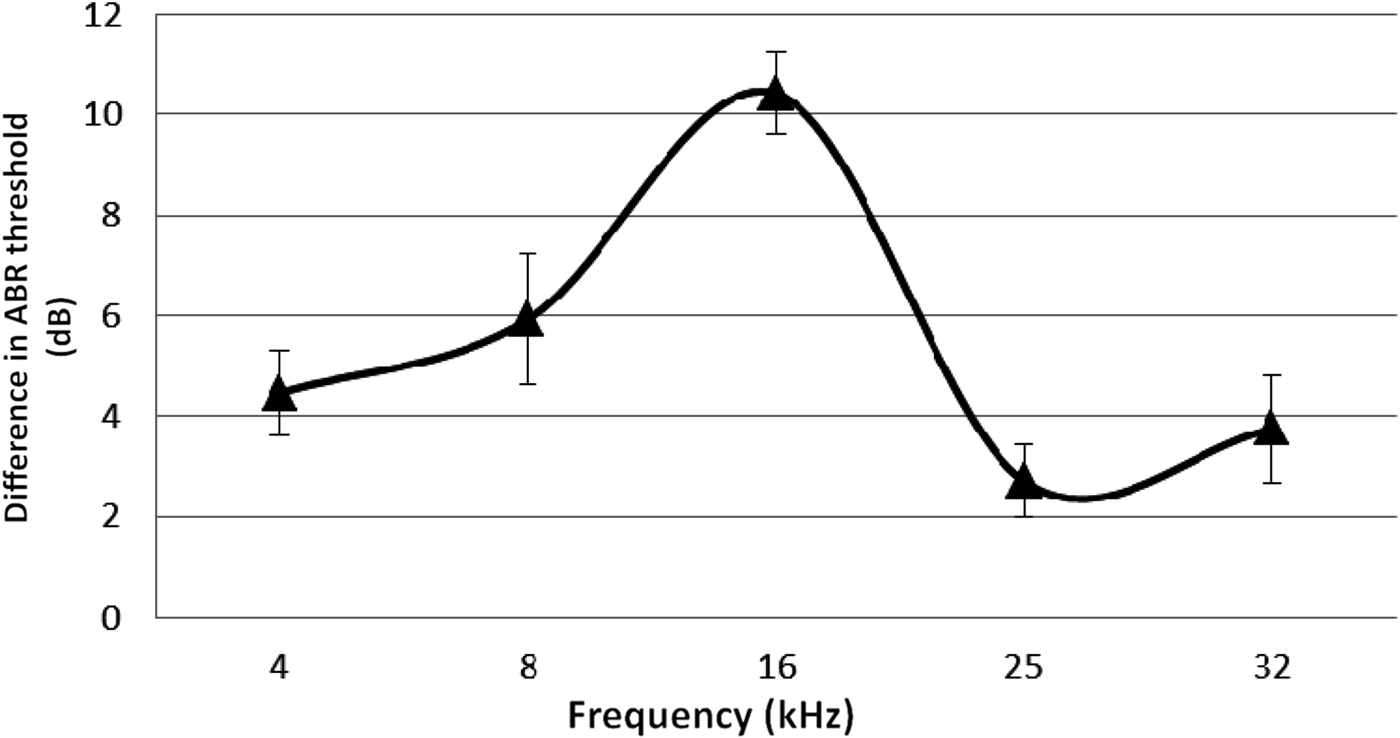
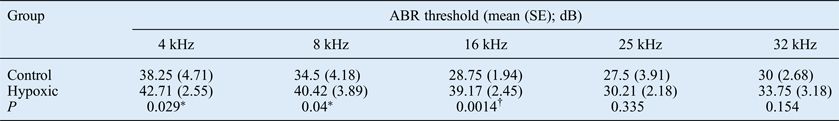
The ABR threshold means from both ears of the hypoxic rats were compared with the means obtained from the control rats. The mean ABR thresholds were higher for the hypoxic rats at all frequencies tested (Figures 1 and 2).

Fig. 1 Mean auditory brainstem response (ABR) thresholds at the different test frequencies in the hypoxic and control ears. Error bars indicate the standard error of the mean.

Fig. 2 Difference in mean auditory brainstem response (ABR) thresholds for different test frequencies, comparing the hypoxic and control ears. Error bars indicate standard error of the mean.
The statistical significance of the inter-group differences in ABRs was determined using the paired t-test. This showed that the overall difference between the control and hypoxic ears was statistically significant (t = −4.05, degrees of freedom = 4, p = 0.016). The significance of differences at each frequency was also obtained, as shown in Table I. The 95 per cent confidence intervals were calculated from the summarised data of the mean values obtained from both groups (Table I).

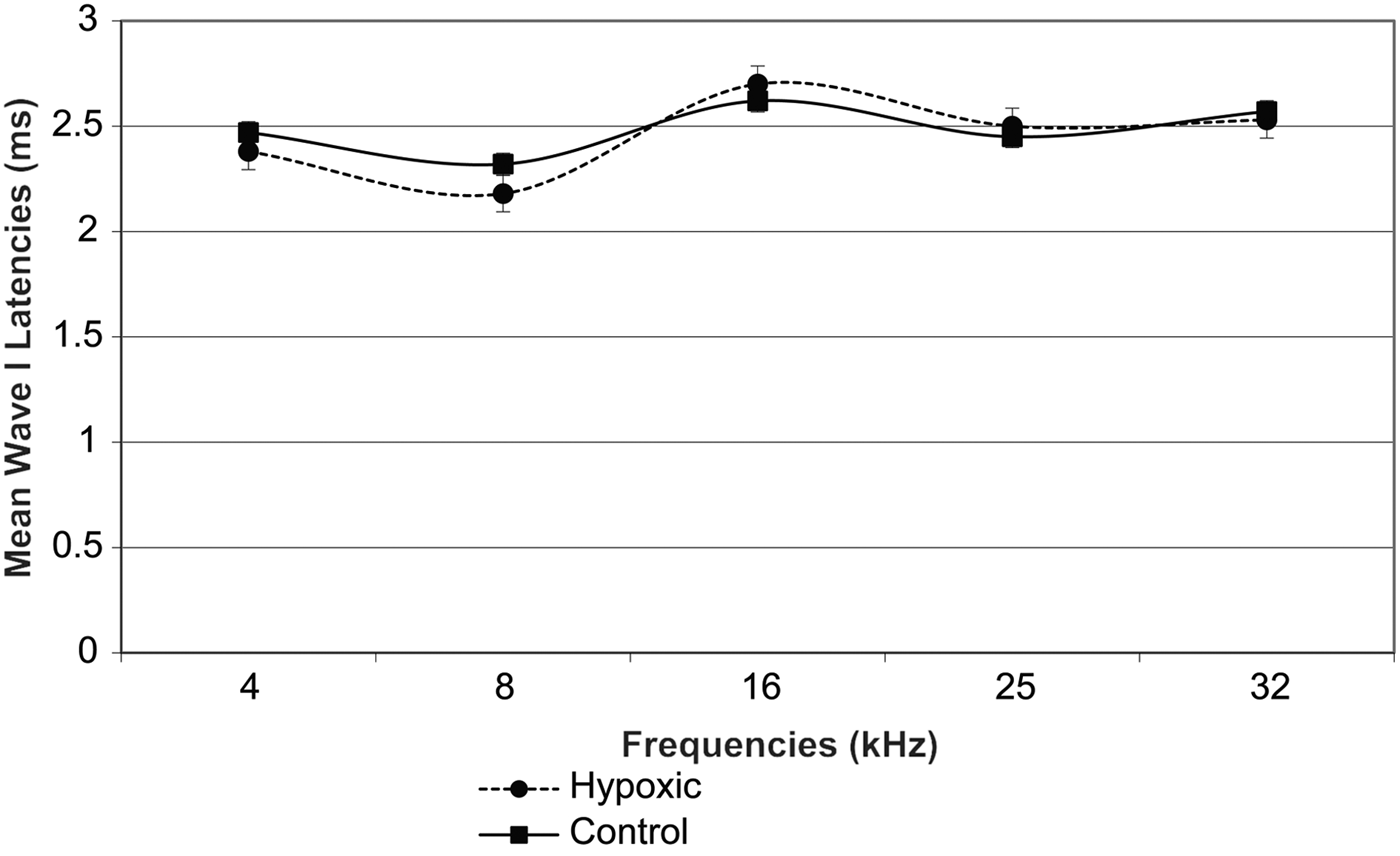
Fig. 3 Mean wave I latencies in the right ears of experimental and control animals. Error bars indicate standard error of the mean.
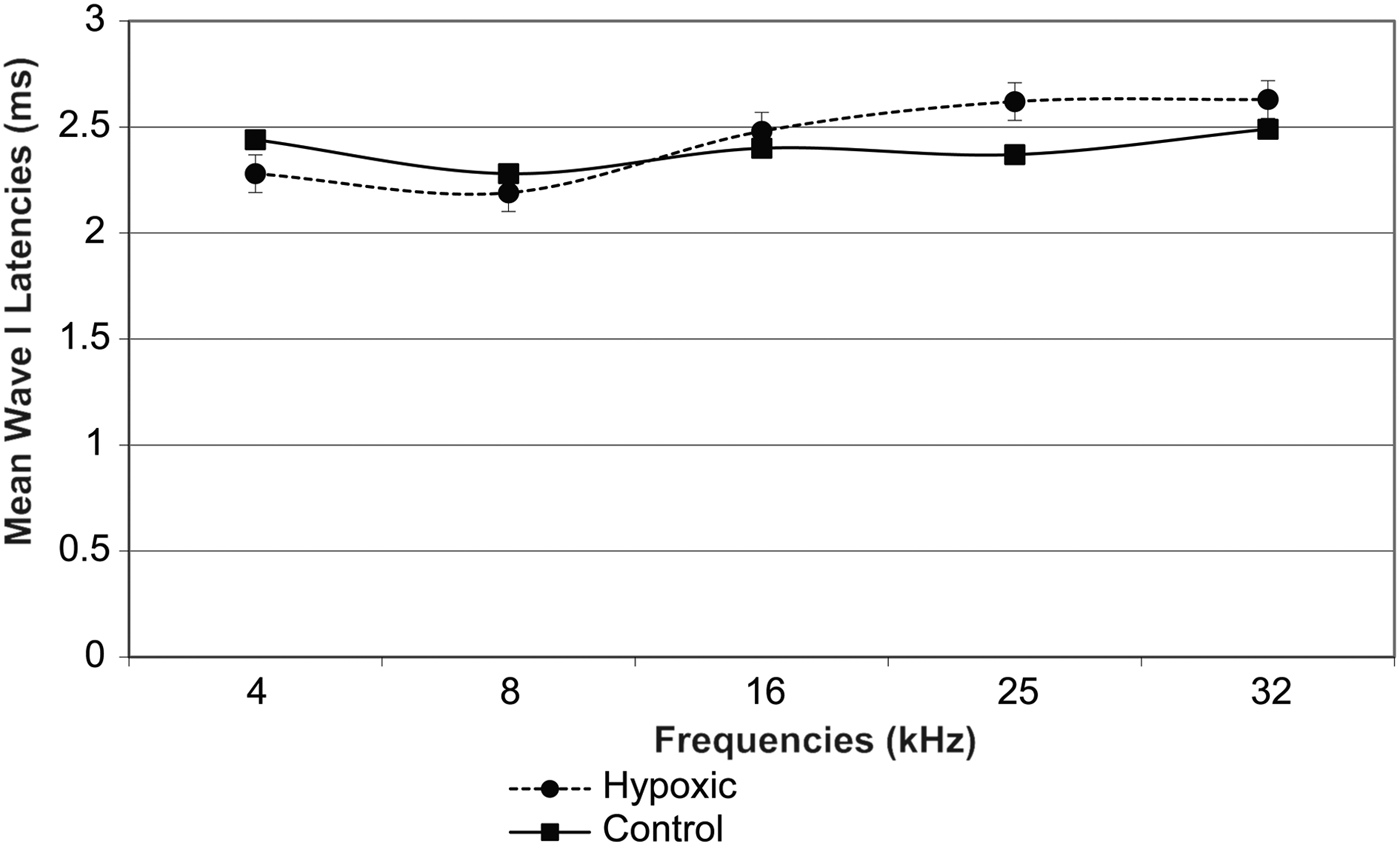
Fig. 4 Mean wave I latencies in the left ears of experimental and control animals. Error bars indicate standard error of the mean.
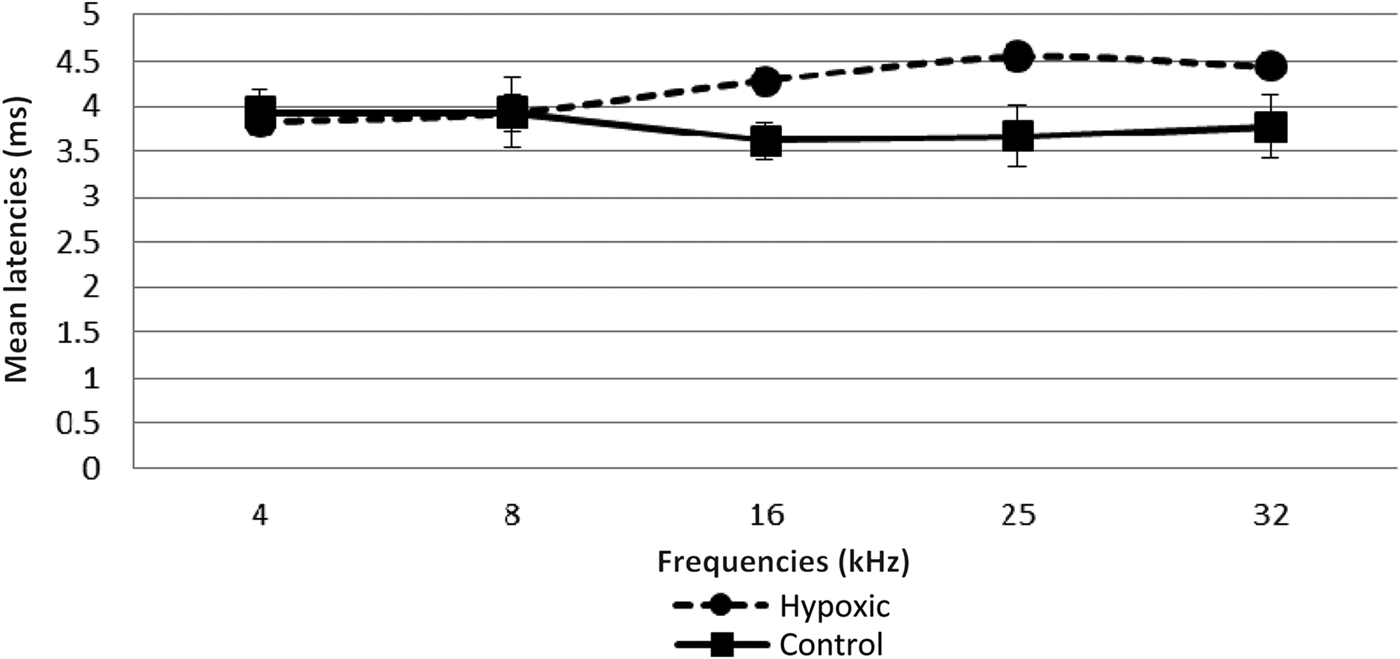
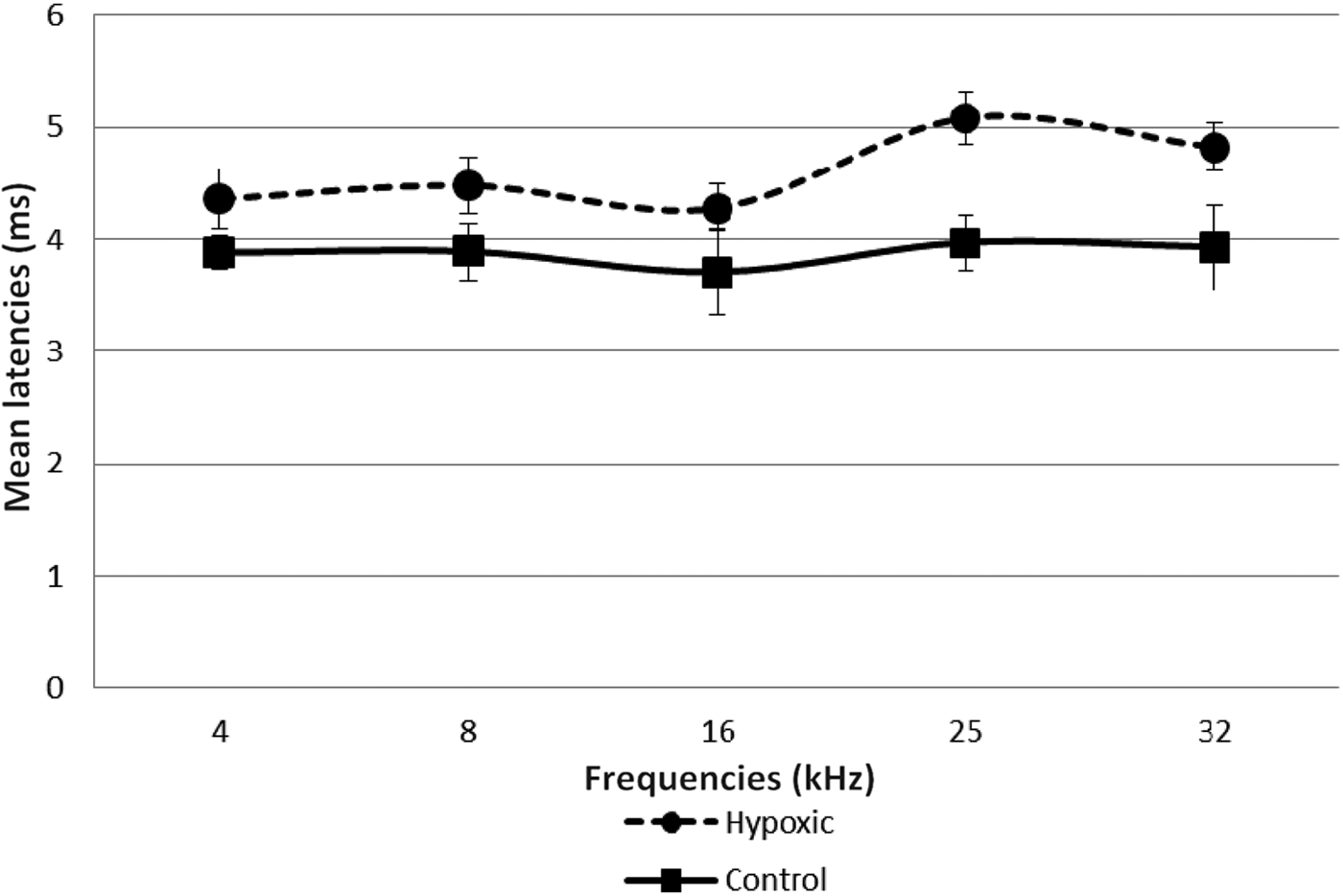
Fig. 5 Mean wave I–V inter-peak latencies in right ears of experimental and control animals. Error bars indicate standard error of the mean.

Fig. 6 Mean wave I–V inter-peak latencies in left ears of experimental and control animals. Error bars indicate standard error of the mean.
Table I Auditory brainstem response threshold data by test frequency and group

For overall data, two-tailed p = 0.0378. Ninety-five per cent confidence interval for summarised mean data for both groups = −11.1292 to −0.6258. Statistical significance: *p < 0.05; †p < 0.01. ABR = auditory brainstem response; SE = standard error
A comparison of the latencies for wave I in the right and left ears of both groups of animals showed no statistically significant differences (Table II, and Figures 3 and 4). However, there was a remarkable increase in the mean wave I–V inter-peak latencies in hypoxic ears compared with the control group (Figures 5 and 6). Statistical analysis indicated that this difference was significant (Table II).
Table II Auditory brainstem response latency data: significance of inter-group differences by test frequency

Data represent p values for comparison of study versus control groups. Statistical significance: *p < 0.01; †p < 0.001; ‡p < 0.05.
Discussion
There is evidence that the development and maturation of the cochlea continues in postnatal life.Reference Birnholz and Benacerraf11, Reference Romand, Despres and Giry12 Perinatal factors that have the potential to influence cellular function may therefore affect the organ of hearing.
A number of studies have investigated the effects of acute hypoxia on the anatomical structure of the cochlea in vitro.Reference Mazurek, Winter, Fuchs, Haupt and Gross4 Other studies, using limited post-mortem human neonatal samples, have shown the impact of perinatal hypoxia on cochlear histology.Reference Birnholz and Benacerraf11 An even smaller number of studies have investigated the effects of perinatal hypoxia and/or ischaemia on children after a prolonged period (six months or one year after birth).Reference Ide, Harada, Kanzaki, Saito, Hoshikawa and Kawahara6–Reference Jiang, Yin, Shao and Wilkinson8
In this study, we utilised an in vivo rat model of postnatal hypoxia and looked at the physiological effects of exposure to hypoxia on hearing at maturity.
Human and animal studies suggest that acutely induced hypoxia results in transiently elevated hearing thresholds that tend to revert back to normal once normoxia is re-established.Reference Cycowicz, Schmuel, Freeman, Wanszelbaum and Sohmer13 The duration of exposure to hypoxia and the animal model studied seem to have an impact on whether or not there will be permanent hearing loss.Reference Gafni and Sohmer14 Earlier studies have suggested that hypoxia-induced hearing is short-lived, especially if it is acute.Reference Cycowicz, Schmuel, Freeman, Wanszelbaum and Sohmer13 The cochlea in animals is more immature at birth; this may explain its vulnerability to perinatal hypoxia. Studies have shown that human newborns too have immature endocochlear potential responses, which accounts for their increased hearing thresholds at birth.Reference Geal-Dor, Freeman, Li and Sohmer15, Reference Kusakari, Kambayashi, Kobayashi, Rokugo and Kawamoto16
The ABR results of the present study demonstrated that the hearing thresholds for all frequencies tested were higher for the animals exposed to postnatal hypoxia compared with the control animals. However, these effects appeared to be more marked at the lower frequencies (4, 8 and 16 kHz); smaller effects were recorded at 25 and 32 kHz, as shown in Figure 2. This apparent pattern of a greater decrease in hearing function with decreasing frequencies secondary to hypoxia has also been shown in clinical studies on newborns who suffered perinatal hypoxia. In those studies, greater hearing effects were reported at lower frequencies (1–5 kHz).Reference Zang, Wilkinson and Jiang7, Reference Jiang, Yin, Shao and Wilkinson8 Similarly, among newborns who suffered perinatal hypoxia, reductions in distortion product otoacoustic emission (DPOAE) amplitudes were observed at frequencies between 1 and 10 kHz, although this was most noticeable at 1–2 kHz.Reference Ide, Harada, Kanzaki, Saito, Hoshikawa and Kawahara6 In a guinea pig model of acutely induced hypoxia, Pierson and Moller found frequency-dependent shifts in latency during hypoxia exposure, with lower frequency clicks exhibiting a greater decrease in latency than higher frequency clicks.Reference Pierson and Moller17 It is not clear why the effects of hypoxia seem to be greater for lower frequencies. A probable reason is the differential vulnerability of different regions of the cochlea to hypoxia.Reference Mazurek, Winter, Fuchs, Haupt and Gross4, Reference Koga, Hakuba, Watanabe, Shudou, Nakagawa and Gyo18 It has been suggested that the increased thresholds in hypoxic ears are induced by the cellular effects of hypoxia on the inner and outer hair cells of the cochlea.Reference Khan, Szczepek, Haupt, Olze and Mazurek19 These sensory cells, as well as the structures of the lateral wall of the cochlea, are critically affected by hypoxia. Although these findings were observed in vitro, they are confirmed by our in vivo animal model.
The prolonged wave I–V inter-peak latencies observed in this study in the hypoxic animals suggest that the central auditory mechanism may also be involved. However, in contrast to the threshold effects, these latency effects were more pronounced for the higher than the lower frequencies.
The current study explored the long-term effects of chronic postnatal hypoxia on hearing function, for which an animal model of long-term hearing outcome after chronic postnatal hypoxia was created. Our findings were similar to those observed by Zang et al., who examined DPOAEs in children with hypoxia-ischaemia and those born with low Apgar scores.Reference Zang, Wilkinson and Jiang7 Although the method of hearing assessment differed and different parts of the auditory pathway were examined, the similarity in the results obtained suggest that long-term effects of hypoxia occur both at the sensory outer hair cell and the auditory brain-stem levels.
• Perinatal hypoxia is one of the known risk factors for hearing loss occurring in the newborn
• In this Sprague–Dawley rat model, chronic perinatal hypoxia induced permanent loss of hearing
• The findings suggest functional abnormality in the central auditory pathway
The method of inducing hypoxia varies across studies. In the current study, we induced hypoxia by rearing rat pups in a mixture of air and nitrogen (to obtain an FiO2 value of 0.12) for 10 days. Hearing assessment was delayed until maturity (approximately 72 days of age). Caution must be used when extrapolating these data directly to humans. Nevertheless, this study provides evidence that chronic hypoxia does permanently affect the cochlea and perhaps also the central auditory pathway.
Conclusion
In our Sprague–Dawley rat model, early life exposure to chronic hypoxia resulted in permanent hearing loss that was greater for frequencies 16 kHz and lower, and was associated with prolonged wave I–V inter-peak latencies.