Introduction
X-linked hypophosphataemic osteomalacia (OMIM+ 307800) (=Online Mendelian Inheritance in ManTM, http://www.ncbi.nlm.nih.gov) is the most common of the genetically determined forms of osteomalacia. It is characterised by reduced tubular reabsorption of phosphate, and is most frequently inherited as an X-linked dominant trait. Other forms, such as an autosomal dominant variant, are very rare.
Hypophosphataemia persists throughout life and may be the only symptom. In childhood, bone deformities and growth retardation are also common.Reference Davies, Kane and Valentine1, Reference Rauch and Schoenau2 In older patients, extraskeletal bone formation occurs in ligaments and tendons.Reference O'Malley, Adams, Davies and Ramsden3 In 1995, X-linked hypophosphataemic osteomalacia was discovered to be caused by mutations in the PHEX gene, which is involved in protein degradation.Reference Consortium4
The association between X-linked hypophosphataemic osteomalacia and hearing loss was first reported in 1984.Reference Davies, Kane and Valentine1 Twenty-five patients from 16 families were studied. Nineteen patients (76 per cent) had a hearing impairment of greater than 15 dB. Sixteen patients were considered to have sensorineural hearing loss and three to have mixed conductive–sensorineural hearing loss. The hearing loss affected exclusively the high frequency range in five patients and both the high and low frequencies in 14. The results of caloric testing showed reduced vestibular function in about 50 per cent of patients.
Meister et al. reported a similar study on 19 patients.Reference Meister, Johnson, Popelka, Kim and Whyte5 They found a measurable hearing loss in three out of 19 patients (16 per cent). All three showed a mild, bilateral sensorineural hearing loss, affecting the high frequencies in two cases and the low frequencies in one.
Both studies concluded that the origin of the hearing loss in X-linked hypophosphataemic osteomalacia was at the level of the cochlea, according to the results of audiometric evaluation, which included measurement of otoacoustic emissions and auditory evoked potentials. The pathological mechanism leading to cochlear hearing loss remains unclear. On the basis of osteosclerosis of the petrous bone, seen on radiographic imaging, it was postulated that such cochlear hearing loss was caused by structural changes, which either alter the metabolism of the stria vascularis, or compress the endolymphatic sac or duct and thus produce an endolymphatic hydrops.Reference Davies, Kane and Valentine1, Reference O'Malley, Adams, Davies and Ramsden3
We present the case of a patient suffering from X-linked hypophosphataemic osteomalacia and hearing loss. For the first time, audiological follow up over a three-year period is reported, as well as the outcome of steroid therapy.
Case report
History
A 55-year-old man presented in December 2003 complaining of fluctuating hearing on the left side, with deterioration on the right side one month later. He also described loud tinnitus occurring periodically on both sides. Dizziness, otalgia and otorrhoea were not reported.
The patient had been known to suffer from X-linked hypophosphataemic osteomalacia since birth. Apart from hearing loss, he did not have any other complaints, and was taking no medication related to X-linked hypophosphataemic osteomalacia.
Laboratory examinations revealed a slightly raised alkaline phosphatase concentration (165 U/l; normal range ≤122 U/l), and no indications of infection.
Initial otological and audiometric findings
The external auditory meatus, the tympanic membrane and the tuning fork tests were normal. Pure tone audiography showed a symmetrical, moderate to severe, sensorineural hearing loss predominantly in the low and high frequency range (Figure 1). The tympanogram and the stapedius reflex thresholds were normal. Auditory evoked potential audiometry showed a clear V wave within normal latencies. Wave I was not identifiable, indicating a cochlear rather than retrocochlear pathology. The patient refused vestibular examination.
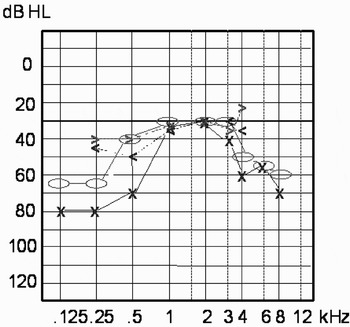
Fig. 1 Pure tone audiogram on first consultation, showing symmetrical, bilateral, moderate to severe hearing loss in the low and high frequency ranges. Bone conduction: o = right, x = left; air conduction: >= right, <= left.
Radiology
A narrowing of the internal auditory meatus and modest sclerosis of the petrous bone were seen on high resolution computed tomography (CT) scanning (Figure 2). Vascular pathology and an intracranial mass were ruled out by magnetic resonance imaging (MRI) with MR angiography.

Fig. 2 High resolution, axial computed tomography scan of the petrous bone, showing narrowing of the internal auditory meatus (arrows) and modest sclerosis of the petrous bone.
Progression
The patient was initially commenced on a decreasing dosage of prednisone (initially 60 mg per day for four days, decreasing by 10 mg every second day for 14 days in total). On completion of this therapy, the patient's hearing threshold levels showed a marked improvement of 20–40 dB HL in the low and middle frequencies (Figure 3), although mainly on the left side. Otoacoustic emissions, measured at the same time as the audiogram shown in Figure 3, were not detectable.
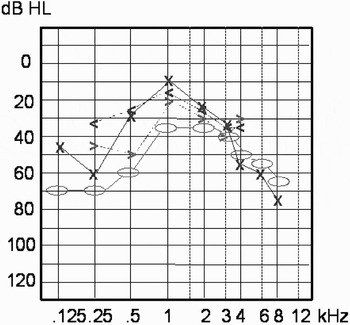
Fig. 3 Pure tone audiogram after completion of initial steroid therapy, showing improved hearing threshold mainly in the low frequencies. Bone conduction: o = right, x = left; air conduction: >= right, <= left.
This improvement lasted for four weeks. No change of tinnitus was reported.
Over the next six months, fluctuation of hearing threshold level and tinnitus was observed, with deterioration of hearing in the low and middle frequency ranges (Figure 4). A maintenance dose of steroids was proposed to the patient but refused.
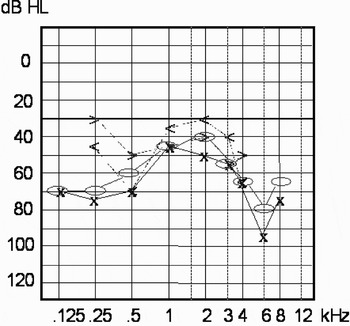
Fig. 4 Pure tone audiogram after six months, showing further hearing deterioration in the middle frequencies. Bone conduction: o = right, x = left; air conduction: >= right, <= left.
The hearing threshold level stabilised spontaneously one year after the onset of symptoms. At this time, pure tone audiography showed a mild to moderate hearing loss with markedly improved hearing in the low and middle frequency ranges of the left ear. The hearing threshold level of the right ear remained almost unchanged (Figure 5).

Fig. 5 Pure tone audiogram after 18 months, showing mild to moderate hearing loss with markedly improved hearing in the low and middle frequency ranges of the left ear. The hearing threshold level in the right ear has remained almost unchanged. Bone conduction: o = right, x = left; air conduction: >= right, <= left.
A sudden hearing loss in both ears occurred almost two years after the onset of symptoms. Unfortunately, the patient did not present immediately, so no audiogram was performed at that time. At the consultation four weeks later, hearing in the left ear had completely recovered, while hearing in the right ear remained decreased by about 10–15 dB (Figure 6). Repeated treatment with steroids was refused by the patient. Over the whole follow-up period, no medication for X-linked hypophosphataemic osteomalacia was administered. Disease markers and clinical examination did not show any progression of X-linked hypophosphataemic osteomalacia in the patient.

Fig. 6 Pure tone audiogram after 24 months, showing an almost unchanged hearing threshold level in the left ear and a decrease in the right ear of 10–15 dB HL. Bone conduction: o = right, x = left; air conduction: >= right, <= left.
Discussion
The literature describing X-linked hypophosphataemic osteomalacia and hearing loss is scarce and outdated. In particular, progression of hearing impairment and possible therapeutic strategies are not described. The audiological phenotype of patients with X-linked hypophosphataemic osteomalacia varies widely. Hearing is affected mainly in the high frequencies in about 45 per cent, in the low frequencies in about 5 per cent and in both frequency ranges in 50 per cent. Hearing loss has been consistently attributed to a cochlear source. The degree of hearing loss ranges from mild to severe.Reference Davies, Kane and Valentine1, Reference Meister, Johnson, Popelka, Kim and Whyte5 Our patient suffered from a mild to moderate sensorineural hearing loss mainly in the low and high frequency ranges. Auditory evoked potentials did not show any neuroconduction impairment. All these observations are in accordance with the literature.
Our patient's substantial improvement after initial steroid therapy was asymmetrical, limited to the low and middle frequencies, and only lasted for four weeks. Of course, the possibility of a spontaneous recovery unrelated to therapy cannot be excluded. Over the following months, the extent of the hearing threshold fluctuation corresponded to the improvement achieved by therapy. However, in contrast, the patient's hearing remained stable in the first four weeks after completion of therapy. After one year, a spontaneous and lasting stabilisation occurred in the left ear. The previously documented low frequency hearing loss recovered. In summary, over the observation period, progression of hearing loss was characterised by fluctuations. While some recovery was seen on one side, the other side stabilised at a mild to severe level of hearing loss.
Our patient's fluctuating hearing, mainly affecting the low frequencies, supports the hypothesis of an underlying endolymphatic hydrops as the cause of hearing impairment. Davies et al. performed electrocochleography in two patients and demonstrated an endolymphatic hydrops.Reference Davies, Kane and Valentine1 This examination was refused by our patient. The progression and audiometric results of our patient are comparable to M Menière, although he has never complained of vertigo.
The alternative theory, postulating neural compression or narrowing of the endolymphatic sac, seems rather unlikely. While Davies et al. described a normal internal auditory meatus,Reference Davies, Kane and Valentine1 O'Malley et al. reported generalised osteosclerosis and narrowing of the internal auditory meatus.Reference O'Malley, Adams, Davies and Ramsden3 Still, both groups postulated that hearing loss was of cochlear origin, based on their audiometric results.Reference Davies, Kane and Valentine1, Reference O'Malley, Adams, Davies and Ramsden3 Our patient's CT scans showed mild osteosclerosis of the petrous bone and some narrowing of the internal auditory meatus, thus refuting the compression theory. In addition, cerebral MRI and auditory evoked potentials did not indicate any retrocochlear lesion.
• X-linked hypophosphataemic osteomalacia is the most common of the genetically determined forms of osteomalacia
• Hearing loss in this condition is characterised by repeated episodes of sudden decrease, predominantly in the low and high frequencies
• While hearing may recover, a stabilisation occurs at around 60 dB HL after a few years. Steroids may have some therapeutic benefit
The therapy used for our patient also supports the theory of an endolymphatic hydrops as the underlying pathogenetic mechanism of hearing loss in X-linked hypophosphataemic osteomalacia. Prednisone is used in the therapy of various cochleo-vestibular diseases, utilising various mechanisms,Reference Plontke6 such as anti-inflammatory and mineralocorticoid receptor mediated effects, especially in the stria vascularis. In addition, glucocorticoids are involved in the homeostasis of the inner-ear fluids by regulation of aquaporines, membrane channels responsible for water transport.Reference Plontke6
An additional line of evidence for the proposed pathogenetic mechanism and beneficial effect of steroids stems from molecular genetic studies. Most studies on the mouse model of X-linked hypophosphataemic osteomalacia indicate that the renal dysfunction observed is the result of a circulating factor rather than a genetic renal defect.Reference Nesbitt, Coffman, Griffiths and Drezner7 Bearing this in mind, the assumed circulating factor causing X-linked hypophosphataemic osteomalacia may precipitate in the stria vascularis, thereby disturbing the metabolism of the stria vascularis, either by local inflammatory changes or as a result of the precipitate itself. This hypothesis is further supported by the other mouse model (Gy phenotype), in which histological examination points to a degenerative process in the cochlea.Reference Boneh, Reade, Scriver and Rishikof8, Reference Francis, Strom, Hennig, Boddrich, Lorenz and Brandau9 The histological finding of degenerate hair cells corresponds to the absent otoacoustic emissions seen in our patient. Steroids would have a positive impact on local inflammatory changes as well as on the disturbance of homeostasis. These two beneficial effects resulted in steroids being the first choice of therapy for our patient, compared with other possible treatments for endolympatic hydrops, such as diuretics.
The treatment of X-linked hypophosphataemic osteomalacia incorporates calcitriol and phosphate salts. However, in our patient's case, use of such agents was precluded by their serious side effects, such as nephrocalcinosis and hyperparathyroidism, and the fact that they have not previously been administered in patients with X-linked hypophosphataemic osteomalacia and hearing loss.Reference Davies, Kane and Valentine1, Reference O'Malley, Adams, Davies and Ramsden3
Conclusions
Hearing loss in X-linked hypophosphataemic osteomalacia is characterised by repeated episodes of sudden decrease, predominantly in the low and high frequencies. While hearing loss may recover, stabilisation occurs at around 60 dB HL after a few years. Steroids may have some therapeutic benefit.








