Introduction
Cerumen is the brownish yellow, waxy secretion produced by the external auditory meatus. It is largely composed of desquamated sheets of keratinocytes. It also contains lipids and peptides which are secreted by the sebaceous and apocrine glands in the external auditory canal.Reference Guest, Greener, Robinson and Smith1 Cerumen acts as a lubricant and a hydrophobic protective layer on the meatal skin. It also contains lysosomal enzymes and immunoglobulins, which gives it bactericidal properties.Reference Chai and Chai2, Reference Burton and Doree3
Cerumen accumulation is normally asymptomatic. It can however prevent adequate clinical examination and cause conductive hearing loss.Reference Burton and Doree3 Elderly patients are at increased risk of cerumen accumulation, as the sebaceous glands atrophy with age and the cerumen becomes dry.Reference Burton and Doree3 One-third of elderly patients in nursing homes require removal of cerumen every year.Reference Grossan4 Hearing aids prevent the natural extrusion of cerumen, and patients with narrow external auditory canals and dermatological conditions are at increased risk of cerumen accumulation.
There are a range of devices and techniques used to mechanically remove cerumen: syringing, irrigation and suction with magnification.Reference Grossan4 Generally, mechanical removal is a safe procedure; however, there are reports of it causing severe otological trauma.Reference Grossan4
Cerumenolytics are topical agents used to aid cerumen removal. They can be divided into oil-based and aqueous-based agents. Cerumenolytics work by hydrating the desquamated sheets of keratinocytes and by inducing keratolysis, causing disintegration of the cerumen.Reference Hand and Harvey5
A wide range of cerumenolytics are available; however, it remains uncertain which are most effective. Limited clinical trials have been performed, but over 30 years ago (see below), so little current evidence is available. Olive oil and sodium bicarbonate preparations are still recommended in the British National Formulary, as they are simple remedies that do not contain organic solvents, which can irritate the skin of the external auditory canal.6
We aimed to conduct an in-vitro study to determine the most effective cerumenolytic, using over the counter, topical agents.
Method
Cerumen was collected by mechanical removal from patients who attended the otolaryngology out-patient clinic over a 2-week period and who were aged between 19 and 89 years old. Verbal consent for inclusion in the study was obtained. Patients with clinical evidence of external ear disease were excluded.
The collected cerumen was mixed and formed into a homogeneous ball and stored in an air-tight container at room temperature. The cerumen was rolled into a disc 3 mm thick. Smaller discs of cerumen were punched out using the end of an otoscope speculum (5 mm diameter), to create samples of uniform shape and size. These discs were then weighed (using AND model GR120 scales; AND, Tokyo, Japan); discs weighing 0.030 g were selected. Each of these cerumen samples was placed into a tube which contained 5 ml of one of six over the counter cerumenolytic preparations. We tested two oil-based agents (olive oil, and urea hydrogen peroxide 5 per cent in glycerol) and four aqueous-based agents (distilled water, sodium bicarbonate, Sofradex (Sanofi-Aventis, Guildford, UK) and Vistamethasone (Cardinal Health Martindale Products, Brentwood, UK)) (see Table I).
Table I Agents tested

*Dexamethasone 0.05%, framycetin sulphate 0.5% and gramicidin 0.005%. †Betamethasone sodium phosphate 0.1%. No = test number; BP = British Pharmacopoeia
The tubes were stored at room temperature and observed at 30 minutes, 3 hours and 12 hours. Digital photographs were taken to record the degree of cerumen disintegration. An individual blinded to the identity of the individual preparations recorded the degree of cerumen disintegration using a scale adapted from Fraser.Reference Fraser7 The cerumen discs were removed from the solutions at 12 hours, dried at room temperature for 48 hours and then re-weighed. The experiment was then repeated a further two times.
Results
Figure 1 shows photographic evidence of the cerumen disintegration. At 30 minutes (Figure 1a), the aqueous-based agents had caused slight disintegration of the cerumen, while the oil-based agents had not caused any visible change. At 3 hours (Figure 1b), partial disintegration of cerumen was seen in the aqueous-based agents, but no visible change was seen in the oil-based agents. At 12 hours (Figure 1c), the distilled water and the sodium bicarbonate solution had caused substantial disintegration of the cerumen, but the oil-based agents had still not caused any visible change to the cerumen.

Fig. 1 Photographic evidence of cerumen disintegration at (a) 30 minutes, (b) 3 hours and (c) 12 hours. See Table I for identity of numbered solutions.
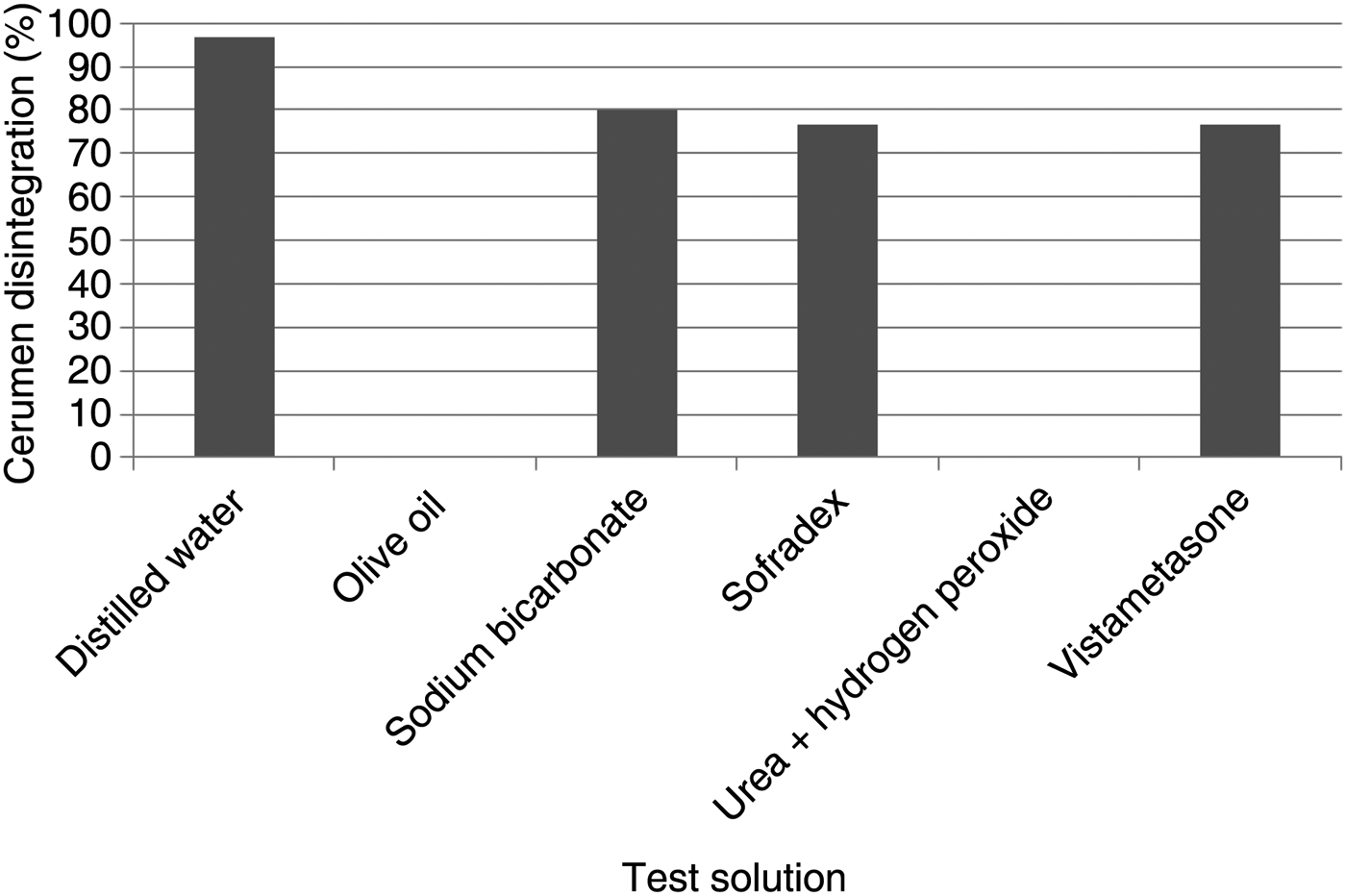
Table II quantifies the degree of cerumen disintegration in the six test solutions, while Table III presents the dried weight of the cerumen disks following exposure to the test solutions. Figure 2 shows the percentage disintegration of cerumen caused by each of the six test solutions.

Fig. 2 Mean percentage cerumen disintegration caused by each test solution.
Table II Degree of cerumen disintegration over time

Adapted with permission.Reference Fraser7 Min = minutes; hr = hours; += slight disintegration; ++ = partial disintegration; ++ + =substantial disintegration; – = no visible change
Table III Dried weight of cerumen

Discussion
In the present study, distilled water was the most effective cerumenolytic as it produced the greatest reduction in the weight of the cerumen test disc. Both the distilled water and the sodium bicarbonate solution caused substantial cerumen disintegration at 12 hours. The two aqueous-based agents had a much greater effect than the four oil-based cerumenolytics. The latter agents resulted in substantially less visible cerumen disintegration, and negligible (if any) decrease in the dried weight of the cerumen disc.
In 2009, a Cochrane systematic review of trials of cerumenolytics identified eight clinical trials, all with small numbers of participants.Reference Burton and Doree3 The reviewers concluded that there was no evidence that any one cerumenolytic was preferable to any other. Mehta (1985), Horowitz (1968) and Fraser (1970) found oil-based cerumenolytics to be less effective than aqueous-based cerumenolytics.Reference Fraser7–Reference Horowitz9 Fraser's in-vivo study found that Cerumol (arachis oil and chlorobutanol; Thornton & Ross, Huddersfield, UK) was a more effective cerumenolytic than sodium bicarbonate; however, Cerumol contains the skin irritants turpentine and dichlorobenzene.Reference Fraser7 Hinchcliffe (1955) noted that Cerumol caused significantly more aural discomfort than other topical preparations.Reference Hinchcliffe10 More recently, Bellini (1989) and Chalishazar (2007) discovered water to be a very effective cerumenolytic.Reference Bellini, Terry and Lewis11, Reference Chalishazar and Williams12
• Topical cerumenolytics are used to aid cerumen removal
• Many types are available but their relative efficacy is uncertain
• In this study, distilled water was the most effective cerumenolytic
• Oil-based cerumenolytics were relatively ineffective
• Use of distilled water as a cerumenolytic would be cheaper for both patients and hospitals
The use of water as a cerumenolytic would have financial benefits for both patients and the National Health Service, reducing both expenditure on cerumenolytics and the time spent mechanically removing cerumen. In addition, mechanical cerumen removal is not without risk. It can be traumatic, causing bleeding of the external auditory canal and perforation of the tympanic membrane, and can also lead to an increased number of infections. Prolonged exposure of the ear to water is associated with otitis externa.Reference Bellini, Terry and Lewis11
The present investigation was limited by being an in-vitro study. The samples were kept at room temperature; however, the physiological temperature of the external auditory canal is higher. This may have affected the cerumenolytic potential of the agents tested. The cerumen samples were from multiple patients, although differences in cerumen consistency were overcome by mixing the difference samples into one homogeneous ball. The study constituted an up to date, experimental trial using a variety of over the counter, topical agents, some of which had not previously been tested.
Conclusion
In the present study, distilled water caused the greatest degree of cerumenolysis. Oil-based cerumenolytics were relatively ineffective. A future, in-vivo study is required to assess the efficacy of distilled water as a cerumenolytic agent and to monitor for any adverse effects.