Introduction
Facial schwannomas are rare, benign tumours of the facial nerve. Despite their rarity, they are the most common tumour of the facial nerve, comprising 64 per cent of tumours in a recent series by Falcioni et al. Reference Falcioni, Russo, Taibah and Sanna1 Facial schwannomas can involve the intracranial, intratemporal or extracranial segments of the facial nerve. This article reviews intratemporal and intracranial facial schwannomas; extracranial schwannomas are not considered, unless they are in continuity with an intracranial or intratemporal tumour.
There are approximately 600 reported cases of intratemporal facial schwannoma in the literature;Reference Perez, Chen and Nedzelski2 hence, most reported series contain relatively small numbers. Kertesz et al. Reference Kertesz, Shelton, Wiggins, Salzman, Glastonbury and Harnsberger3 reported the anatomical and radiological features of a series of 88 facial neuromas taken from the House Ear Clinic and University of Utah Medical Centre, but no clinical or management details were presented. O'Donoghue et al. presented 48 facial neuromas from the Otologic Medical Group.Reference O'Donoghue, Brackmann, House and Jackler4 Falcioni et al. Reference Falcioni, Russo, Taibah and Sanna1 and Kim et al. Reference Kim, Chang, Oh, Ahn, Hwang and Lee5 have each presented series containing 18 cases of facial neuroma.
This series of 53 patients with 53 facial schwannomas was taken from two tertiary referral skull base centres in Sydney, and represents the largest published series of facial schwannomas to be reported with clinical and management considerations.
Facial schwannomas are enigmas, with many contentious issues regarding diagnosis and management. This article will also present a review of the current literature on these topics, and a suggested management algorithm.
Materials and methods
Fifty-three patients with facial schwannomas were treated by the two senior authors in tertiary referral skull base centres from 1985 to 2005. The patients' charts were retrospectively reviewed and data collated. Three patients (6 per cent) were lost to follow up; all remaining patients presented for regular clinical assessment.
Results
Epidemiology
There were 23 (43 per cent) female and 30 (57 per cent) male patients. Patients' ages at presentation ranged from five to 84 years, with a mean of 49 years. Twenty-five (47 per cent) of the tumours were present on the left side and 28 (53 per cent) on the right side.
Clinical presentation
The presenting symptoms are summarised in Table I. The most common presenting symptom was hearing loss, being present in 31 of the 53 (58 per cent) patients. Eighteen patients (34 per cent) had a sensorineural hearing loss, eight patients (15 per cent) had a conductive hearing loss and five (9 per cent) had a ‘dead ear’ (Table II).
Table I Clinical presentation

*Of all patients (n = 53). SNHL = sensorineural hearing loss; CHL = conductive hearing loss
Table II Hearing status at presentation

*20–40 dB; †40–60 dB; ‡60–80 dB. CHL = conductive hearing loss; SNHL = sensorineural hearing loss
Facial weakness was present in 27 patients (51 per cent), and was of a recurrent, fluctuating nature in three patients (6 per cent). Facial function was graded using the House–Brackmann system, and the results at initial presentation and final follow up are presented in Table III and Figure 1. Twenty-six patients (49 per cent) had normal facial movements at the time of initial presentation. Of the 27 patients (51 per cent) with reduced facial movements, six (11 per cent) had a complete facial palsy.

Fig. 1 Facial nerve function at initial and final presentations. LTFU = lost to follow up
Table III Facial nerve function at initial and final presentations

*Of all patients (n = 53). HB = House–Brackmann; LTFU = lost to follow up
Tinnitus affected 13 patients (25 per cent). Abnormal facial movements, in the form of tics, twitching or formication, were present in nine patients (17 per cent), and facial synkinesis was present in three patients (6 per cent). Eight patients (15 per cent) had imbalance or ataxia, and five patients had vertigo (9 per cent). Headache or otalgia was present in seven patients (13 per cent). Less common symptoms are detailed in Table I. Interestingly, two patients (4 per cent) were completely asymptomatic, and their facial schwannomas were diagnosed incidentally.
Surgical pathology
Tumour location
The extent of the schwannomas along the facial nerve was determined from a combination of radiological and intra-operative findings (in those cases in which surgery was undertaken). These findings are summarised in Table IV and Figure 2. The schwannoma extended along more than one segment of the facial nerve in 39 patients (74 per cent), and was limited to one segment in 14 patients (26 per cent). The mean number of segments involved was 2.5.

Fig. 2 Facial nerve segments involved with tumour. GSPN = greater superficial petrosal nerve; IAC = internal auditory canal; CPA = cerebellopontine angle
Table IV Tumour extension along facial nerve: segments involved

Total = 52 (as extent of nerve involvement not available for 1 patient (patient 20 PW)).
Histopathology
All surgically treated tumours were diagnosed as schwannomas; there were no malignancies.
Management
Management of these facial neuromas was either via clinical observation or surgical removal. No patient received radiotherapy or radiosurgery.
Observation
A conservative approach of clinical observation was undertaken in 20 patients (38 per cent). Of these 20 patients, three (6 per cent) had been lost to follow up at the time of the study. Interestingly, at the time of writing, none of the remaining 17 patients under observation had subsequently undergone surgery (i.e. after a mean follow up of 27 months) (two patients had been under observation for almost eight years without surgical intervention – patients 24 (MH) and 43 (AY)).
Surgery
Thirty-three patients (62 per cent) underwent surgery, with a total of 36 procedures; the details are outlined in Tables V to VII and Figure 3. Seventeen of the 33 primary procedures were conducted via a translabyrinthine approach, six via a retrosigmoid approach, three via a combined middle fossa–transmastoid approach, three via a combined transmastoid–transparotid approach, two via a transmastoid approach only, one via a middle fossa approach only and one via a transotic approach. The choice of surgical approach was determined by the tumour's size and location and the patient's hearing status. In some cases, a non-hearing-preserving approach was used in the presence of serviceable hearing, where it was felt that a hearing-preserving approach would not provide adequate exposure for tumour removal and nerve reconstruction.

Fig. 3 Surgical approaches.
Table V Management

*Of all patients (n = 53). LTFU = lost to follow up
Table VI Surgical approaches
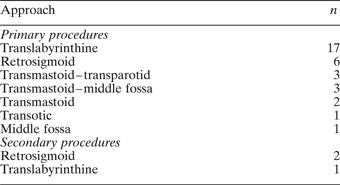
Table VII Tumour removal

*Of patients undergoing surgery (n = 36).
Two patients underwent revision surgery for residual or recurrent disease, with a total of three procedures. One patient initially underwent a retrosigmoid approach and drainage of the cystic component of the tumour, which was repeated four months later, before ultimately requiring a translabyrinthine approach and total tumour removal six months later. Another patient underwent a retrosigmoid approach and drainage of the cystic component of the tumour, which was also repeated.
Three patients underwent surgical exposure of the tumour but no actual tumour removal. These cases were pre-operatively diagnosed as vestibular schwannomas, and were only discovered to arise from the facial nerve intra-operatively. One patient underwent exposure and biopsy of the tumour to exclude malignancy. This did not cause any deterioration in facial nerve function. The tumour was completely removed in 21 cases; the facial nerve was inseparable from the tumour in 20 of these cases, but in one case the tumour was dissected free of the facial nerve, which remained intact. Subtotal tumour removal was performed in four cases. Decompression with no tumour removal was performed in three cases, and drainage of the cystic component of the tumour was performed in two patients on four occasions. The proportions of different surgical procedures are shown in Figure 4.

Fig. 4 Tumour removal.
Nerve reconstruction
Nerve reconstruction was not required in 15 of the 36 surgical procedures. In these cases, the nerve was intact because the tumour had been drained, decompressed, biopsied, left untouched or, as in one patient, the actual tumour had been dissected free of the facial nerve, which remained intact. Twenty-one nerve reconstructions were performed, as detailed in Table VIII and Figure 5. Where the nerve gap was small and the nerve stumps could be easily apposed without tension, an end–end repair using 9/0 nylon sutures was performed. Where the nerve gap was too large for this and the proximal nerve stump was not too close to the brainstem for suturing, an autologous nerve graft was used, harvested from either the greater auricular or sural nerve. Where the proximal nerve stump was too close to the brainstem to allow suturing of a nerve graft, a hypoglosso–facial anastomosis was performed, either at the time of tumour removal or subsequently.

Fig. 5 Nerve reconstruction.
Table VIII Nerve reconstruction methods

*Of patients undergoing surgery (n = 36).
Facial rehabilitation
Eighteen facial rehabilitation procedures were performed on 14 patients (Table IX). A gold weight was inserted into the upper lid of 10 patients (19 per cent), and subsequently removed in two patients. Lateral tarsorraphy was performed on two patients. Dynamic reanimation, using a nerve–muscle pedicled gracilis flap and temporalis rotation graft, was undertaken in two patients.
Table IX Facial rehabilitation surgery

*Of all patients (n = 53).
Outcomes
Surgical complications
There were no complications in the conservatively treated patients. There were five post-operative complications in the 33 patients who underwent surgery (Table X). Only one patient developed a wound infection and cerebrospinal fluid (CSF) leak, necessitating a subtotal petrosectomy and blind sac closure.
Table X Post-operative complications

*Of patients undergoing surgery (n = 36). CSF = cerebrospinal fluid; EAC = external auditory canal
Further surgery was carried out on five patients, for a variety of reasons (Table XI). Two patients underwent ossicular chain reconstruction at a later date, and one patient required reconstruction of the external auditory canal. Finally, a vestibular nerve section was performed in one patient due to intractable vertigo, following translabyrinthine total removal of their facial schwannoma.
Table XI Other surgery

*Of patients undergoing surgery (n = 36). EAC = external auditory canal; CSF = cerebrospinal fluid
Facial nerve outcomes
Seventeen patients were treated conservatively with clinical observation. The time-frame of observation ranged from three to 95 months, with a mean of 27 months. Fourteen of the 17 observed patients did not have any change in their House–Brackmann grade during the period of observation. Three patients had a change in their degree of facial function, and hence in their House–Brackmann grade, during the period of observation: patient 15 (EE) went from grade I to III over 32 months, patient 24 (MH) from grade I to II over 95 months, and patient 45 (AS) from grade II to I (i.e. improvement) over 14 months. The mean value of the initial House–Brackmann grade in the observation group was 1.65, and mean value of the final grade was 1.71. Overall, there was no significant detriment in facial function in those patients treated by observation.
Thirty-one patients underwent surgery. These patients were grouped according to the nature and extent of tumour removal, as shown in Table XII. The mean House–Brackmann facial outcome was 3.0 for end–end anastomoses, 3.8 for greater auricular grafts, 3.63 for sural nerve grafts and 4.6 for hypoglossal–facial anastomoses (4.0 when a jump graft was used). Facial outcomes were better in patients in whom no removal, decompression or subtotal removal was undertaken.
Table XII Extent of tumour removal

HB = House–Brackmann; pre-op = pre-operative; post-op = post-operative; GAN = greater auricular nerve; SN = sural nerve
Table XIII
summarises all the patient data.

Discussion
Epidemiology and aetiology
Schwannomas are the most common tumour involving the facial nerve. In a series of 600 temporal bones, the incidence of intratemporal facial schwannoma was 0.8 per cent.Reference Saito and Baxter6
The results of this case series are generally similar to those of other reported series. There was a slight male preponderance in our 53 patients, whereas Sherman et al. found no gender predilection.Reference Sherman, Dagnew, Pensak, van Loveren and Tew7 The mean age at presentation in our series was 49 years, compared with 40 years in the series reported by Saito and Baxter.Reference Saito and Baxter6 Children are very rarely affected;Reference Sherman, Dagnew, Pensak, van Loveren and Tew7 this was reflected by the presence of only one child in our series.
In our series, there was a slight increase in tumour prevalence on the right side, with 53 per cent of tumours arising from the right facial nerve; this has also been reported previously.Reference Sherman, Dagnew, Pensak, van Loveren and Tew7 Bilaterality is very rare.Reference Fenton, Morrin, Smail, Sterkers and Sterkers8
Unlike vestibular schwannomas, there have been no genetic loci identified as being associated with facial schwannomas. There is an association with neurofibromatosis, but less so for schwannomas than for neurofibromas.
Surgical pathology
Tumour location
The facial nerve contains approximately 10 000 axons: 6000 motor axons, and 4000 parasympathetic, taste and sensory axons. Proximal to the geniculate ganglion, the nerve is devoid of epineurium and is surrounded by CSF; distal to the geniculate ganglion, there is in-growth of connective tissue and separation into fascicles.Reference O'Donoghue, Brackmann, House and Jackler4
According to Kertesz et al., multiple segments of the facial nerve are involved with tumour almost twice as often as single segments (64 and 36 per cent, respectively),Reference Kertesz, Shelton, Wiggins, Salzman, Glastonbury and Harnsberger3 with an average of 2.57 segments involved per tumour.Reference Kertesz, Shelton, Wiggins, Salzman, Glastonbury and Harnsberger3 This compares almost identically to the results of our series, i.e. 2.5 segments per tumour.
In the series of facial nerve tumours reported by Falcioni et al., Reference Falcioni, Russo, Taibah and Sanna1 the geniculate ganglion and tympanic segment were the most common segments containing tumour (75 per cent). Kertesz et al. reported the labyrinthine segment (44 per cent) and geniculate ganglion (51 per cent) to be the most frequently involved segments.Reference Kertesz, Shelton, Wiggins, Salzman, Glastonbury and Harnsberger3 Our results differed slightly; the intracanalicular segment (43 per cent) and geniculate ganglion (45 per cent) were most commonly affected. The greater superficial petrosal,Reference Kumon, Sakaki, Ohta, Ohue, Nakagawa and Tanaka9 stapediusReference Lipkin, Coker, Jenkins and Alford10 and chorda tympaniReference Biggs and Fagan11 branches have also been reportedly involved with schwannoma.
Histopathology
Schwannomas are generally well encapsulated and eccentric to the nerve fascicles, with few axons actually traversing the tumour mass. There are variable amounts of Antoni A and B areas. Antoni A regions are variously organised arrays of spindle cells which in cross-section show a palisading pattern of nuclei termed Verocay bodies. Antoni B regions show loose, hypocellular stroma. Cystic degeneration, haemorrhage and necrosis may be present.
Growth rates
In their series of nine non-vestibular schwannomas, O'Reilly et al. have shown the growth rates of four facial schwannomas to be 0.3, 0.3, 0.1 and 0.01 cm3/year, giving a mean growth rate of 0.18 cm3/year.Reference O'Reilly, Mehanna, Kishore and Crowther12 Perez et al. observed growth in four of 13 facial schwannomas treated expectantly, with a mean growth rate of 1.4 mm/year in the tumours that grew, but a mean growth rate of 0.42 mm/year if all 13 tumours were considered.Reference Perez, Chen and Nedzelski2 Tumour growth rates were not measured in our series.
Malignant potential
Malignant change of schwannomas is rare, with only three cases reported in the literature.Reference Falcioni, Russo, Taibah and Sanna1, Reference Kertesz, Shelton, Wiggins, Salzman, Glastonbury and Harnsberger3 There is a strong association with neurofibromatosis. Histological features of malignancy include the presence of mitoses, pleomorphism and hypercellularity.Reference Muhlbauer, Clark, Robertson, Gardner and Dohan13 Malignant schwannomas rarely metastasise, but if this occurs the lung is the most common site.Reference Muhlbauer, Clark, Robertson, Gardner and Dohan13 These tumours are radio-resistant, with poor chemoresponsiveness. The treatment of choice is radical resection.Reference Muhlbauer, Clark, Robertson, Gardner and Dohan13 None of the surgically treated tumours in our series were malignant.
Clinical presentation
Intuitively, the clinical presentation of facial schwannomas should depend on the location and size of the tumour. Interestingly, Lipkin et al. showed no correlation between tumour size and clinical symptoms.Reference Lipkin, Coker, Jenkins and Alford10 Intracranial and intratemporal facial schwannomas tend to have more severe clinical manifestations than do extracranial facial schwannomas.Reference Chung, Ahn, Kim, Nam, Kim and Lee14
Facial weakness is the most common presenting symptom of facial schwannoma, due to interruption of the motor component of the facial nerve, However, O'Donoghue et al. showed normal facial function in 54 per cent of their series.Reference O'Donoghue, Brackmann, House and Jackler4 Facial weakness may be acute or slow onset. It may be recurrent, and may have associated facial spasms due to irritation of the motor fibres. Facial nerve tumours account for 5 per cent of all cases of facial paralysis, and must therefore be considered in all cases of facial palsy.Reference O'Donoghue, Brackmann, House and Jackler4 These symptoms are more likely when the tumour is present within the fallopian canal,Reference McMenomey, Glasscock, Minor, Jackson and Strasnick15 especially the labyrinthine segment.Reference Sherman, Dagnew, Pensak, van Loveren and Tew7
Conductive hearing loss may occur due to: a mass effect of the tumour within the middle ear (tympanic or mastoid segments); ossicular erosion (tympanic or mastoid segments); or a mass effect within the external auditory canal (mastoid segments). Sensorineural hearing loss may be present if there is cochlear erosion (labyrinthine or tympanic segments), or compression of the cochlear nerve within the internal auditory canal or brainstem (intracranial or canalicular segments). Tinnitus is variably present, probably due to cochlear nerve compression. Vestibular dysfunction can occur, due to labyrinth erosion (tympanic or mastoid segments) or to compression of the vestibular nerves within the internal auditory canal or brainstem (intracranial or canalicular segments).
Otalgia and otorrhoea can occur if the tumour is within the middle ear or external ear canal, in which case a mass or aural polyp may be present. If there is extratemporal extension, there may be a parotid mass. Xerophthalmia and dysgeusia may occur if fibres of the chorda tympani are involved, and hyperacusis if stapedius fibres are affected. Facial numbness can occur if there is compression of the trigeminal nerve within the cerebellopontine angle.
The most common presenting symptom in our series was hearing loss, at 58 per cent, compared with 56 per cent (18 of 32) in McMenomey and colleagues' series of facial nerve tumours.Reference McMenomey, Glasscock, Minor, Jackson and Strasnick15 Most were of a sensorineural nature. Fifty-one per cent of our patients had facial weakness at presentation, compared with 47 per cent of McMenomey and colleagues' series.Reference McMenomey, Glasscock, Minor, Jackson and Strasnick15 Both these incidences are less than the 86 per cent (in 28 facial nerve tumours) reported by Falcioni et al. Reference Falcioni, Russo, Taibah and Sanna1
Imaging
High resolution computed tomography (CT) and magnetic resonance imaging (MRI) scanning with contrast have revolutionised the diagnosis of facial schwannomas. Both modalities are complementary, with MRI allowing superior determination of the nerve of origin and the extent of tumour spread, and CT providing better detail regarding the surrounding bony structures. Facial schwannomas may be seen as a mass within the cerebellopontine angle, internal auditory canal or geniculate ganglion or along the fallopian canal; uncommonly, they may be visible within the middle cranial fossa.Reference Kertesz, Shelton, Wiggins, Salzman, Glastonbury and Harnsberger3 A comma-shaped mass projecting into the cerebellopontine angle may be present, with eccentric tumour projection posterior to the axis of the internal auditory canal.Reference Fagan, Misra and Doust16
Bone-algorithm CT may demonstrate increased width of the fallopian canal, and the presence of a mass expanding or remodelling its bony surrounds rather than aggressively destroying its margins.Reference Kertesz, Shelton, Wiggins, Salzman, Glastonbury and Harnsberger3 Other features evident on CT scans may include a ‘signet ring’ appearance at the geniculate ganglion,Reference Fagan, Misra and Doust16 and otic capsule erosion (present in 29 per cent of O'Donoghue and colleagues' series).Reference O'Donoghue, Brackmann, House and Jackler4 On MRI scanning, facial schwannomas are hypo-intense or iso-intense relative to brain tissue, and enhance following gadolinium administration, although normal facial nerves may also enhance following gadolinium administration. Heterogeneity or cystic change is best appreciated on T2-weighted images.Reference Kertesz, Shelton, Wiggins, Salzman, Glastonbury and Harnsberger3 Skip lesions may be present.
Facial schwannomas restricted to the cerebellopontine angle and/or internal auditory canal, without extension to the labyrinthine segment or beyond, can be mistaken for vestibular schwannomas. Involvement of the geniculate ganglion or labyrinthine segment is perhaps the best way to distinguish facial from vestibular schwannomas. Additional clues may be obtained from the clinical history: a patient with facial palsy and a small cerebellopontine angle or intracanalicular tumour probably has a facial rather than a vestibular schwannoma. Despite this, some authors believe that MRI can differentiate facial schwannomas from vestibular schwannomas and other internal auditory canal lesions (such as haemangiomas).Reference O'Donoghue, Brackmann, House and Jackler4, Reference Sherman, Dagnew, Pensak, van Loveren and Tew7, Reference Fagan, Misra and Doust16
Electrophysiology
The role of electrophysiological tests in the diagnosis and surveillance of facial schwannomas is limited. Electroneurography (ENoG) provides indirect information regarding the number of synchronous functional units of motor axons, motor end-plates and muscle fibres which remain, but it has limited value in slowly progressive lesions with partial degeneration and regeneration,Reference Lipkin, Coker, Jenkins and Alford10 which is the case in many facial schwannomas. It may be used as an adjunct to clinical tests of facial function, supplying additional details on the progression of facial weakness and thus supplementing House–Brackmann classification. In theory, this could provide a useful comparison, from one appointment to the next, in those patients under observation who are deteriorating but not significantly enough to change their House–Brackmann grade. However, in reality, ENoG is rarely used in this setting. Electroneurography has also been used in conjunction with auditory brainstem response testing to differentiate facial from vestibular schwannomas,Reference Brackmann, House, Selters, Graham and House17 although this capability is now less often required, due to improved imaging techniques.
Biopsy
When facial schwannomas are encountered unexpectedly at the time of surgery for presumed vestibular schwannoma, the surgeon is faced with the dilemma of how to proceed. Biopsy would confirm the diagnosis of schwannoma and exclude malignancy; however, malignancy is exceedingly rare, and there is a significant risk of damaging facial nerve fibres, which may be scattered within the tumour.Reference Hajjaj and Linthicum18 Biopsy is generally contraindicated unless malignancy is suspected, such as in patients with neurofibromatosis.
Management
Whether to operate or observe?
It is generally agreed that surgery is the mainstay of treatment of facial schwannomas when intervention is required. There is very little published information on the use of radiotherapy for facial schwannomas: one study reported a series of two patients treated with stereotactic radiosurgery, with control of tumour growth during follow-up periods of 29 and 56 months.Reference Sherman, Dagnew, Pensak, van Loveren and Tew7
The issue of when to surgically intervene is perhaps the most contentious of all, but should be prefaced by the question of why to surgically intervene. Not all facial nerve lesions require surgical management. Suspicion of malignancy and neurofibromatosis type oneReference McGuirt, Johnson and McGuirt19 are rare situations but would strongly indicate surgery. Unlike vestibular schwannomas, brainstem compression and hydrocephalus are uncommon with facial schwannomas, but when they occur surgery is again strongly indicated. The main reason to operate is to halt or slow the deterioration in facial function, or, in advanced lesions, to remove the tumour and reconstruct the nerve to provide the optimal level of recovery of facial function.
Facial schwannomas are almost always slow-growing,Reference Kertesz, Shelton, Wiggins, Salzman, Glastonbury and Harnsberger3, Reference Saito and Baxter6 and facial function can remain stable for long periods. An expectant approach is appropriate in reliable patients with no or mild facial weakness who can attend regular follow-up appointments, although this is not always possible. Observation for a period of time before surgery does not result in worse facial outcomes, as detailed in an earlier review by Liu et al. which assessed some of the patients from within this present study group.Reference Liu and Fagan20
When to operate?
Early surgery has the potential advantage of allowing nerve regeneration to occur when the number of cell bodies within the facial motor nucleus is greatest, and motor end-plates and muscle fibres are best preserved. However, patients with House–Brackmann grades I or II may maintain this level of function for years, whereas early surgery commits them to an immediate grade VI level, and ultimately to a likely grade III or IV level of function. Hence, early surgery has potentially ‘cost’ these patients years of better functionality.
Late surgery is similarly disadvantageous. If surgery is performed when most facial function is lost (i.e. House–Brackmann grades V or VI), there are fewer cell bodies remaining within the facial motor nucleus, and the motor end-plates and muscle fibres will have degenerated significantly. The functional outcome will be poor.
The duration of pre-operative facial paralysis is a very important predictor of post-operative facial function,Reference Kim, Chang, Oh, Ahn, Hwang and Lee5 and was the most important factor in one series.Reference O'Donoghue, Brackmann, House and Jackler4 Falcioni et al. advocate surgery within the first year after the beginning of deterioration in facial function.Reference Falcioni, Russo, Taibah and Sanna1 Better functional results occur when the pre-operative deficit is less; a House–Brackmann grade III or IV level will have better results than a grade V or VI level.Reference Kim, Chang, Oh, Ahn, Hwang and Lee5
The optimal time for surgical intervention is a compromise between these two extremes. As soon as facial function has degenerated to House–Brackmann grade III, which is the equivalent level of the best functional outcome of surgical removal and repair, then surgery must be contemplated. Certainly, once facial function has degenerated to House–Brackmann grade IV, the time for surgical intervention has come.
How to operate?
Once the decision has been made to surgically intervene, several factors must be considered in choosing the surgical approach and technique. Patient factors include age, general level of fitness, co-morbidities, patient personality and audiovestibular function. Disease factors include size and location of tumour, presence of compression of surrounding structures, degree of facial movement, duration of the lesion, and growth rate.
Several surgical approaches are available: transmastoid, translabyrinthine, transotic, transcochlear, retrosigmoid, middle fossa, transparotid or combinations of the above. The surgical approach used will reflect the patient and disease factors outlined above and the training and philosophy of the surgeon, as well as the surgeon's intention – conservative or aggressive.
Prior to widespread use of high resolution CT and MRI imaging with contrast, facial schwannomas were frequently diagnosed unexpectedly at the time of surgery for presumed vestibular schwannoma. A management dilemma then ensued, with the surgical options being to abort the procedure, biopsy the tumour to exclude malignancy, or proceed to subtotal or total removal. Invariably, patients had been prepared for vestibular schwannoma removal, and may not have been informed or given their consent appropriately for the issues associated with facial schwannoma removal. Consequently, no tumour removal or a limited biopsy was performed, and the tumour left in situ, especially if facial function was normal or only mildly reduced. This may have spared facial function for a longer period of time, but often necessitated subsequent surgery. This situation is now less likely due to improved imaging techniques and heightened awareness of facial schwannomas, but it may still occur when the tumour involves only the cerebellopontine angle and/or intracanalicular segments.
A surgical procedure not involving tumour removal is also an option. Conservative surgical procedures aim to halt the deterioration of facial function and to postpone definitive resection and reconstruction, prolonging native facial function as long as possible, perhaps indefinitely. If a cystic component of a facial schwannoma is present, drainage may halt the progression of deterioration in facial function for several years, and may even be repeated if required (as previously detailed by Rodrigues et al., in a report analysing some of the same patients within this present series).Reference Rodrigues, Fagan and Biggs21 There were four drainage procedures performed in our series. Facial schwannoma decompression involves widening of the narrow confines of the fallopian canal without tumour removal.Reference Saito and Baxter6 Decompression without tumour removal was performed in three patients in our series, with a mean improvement in House–Brackmann grade from 2.67 to 1.67 post-operatively.
Subtotal tumour removal can preserve facial function, but leaves tumour behind, in a similar fashion to vestibular schwannoma surgery. In deciding when to cease tumour removal, the surgeon is guided intra-operatively by responses to facial nerve monitoring equipment. The fate of the tumour remnant is variable; it may atrophy, remain unchanged or grow. Should subsequent growth of the tumour remnant occur, further surgery is required which entails ultimately worse facial function.
In our series, four patients underwent subtotal removal of tumour, with maintenance of a mean pre-operative House–Brackmann grade of 1.5 post-operatively.
When complete tumour removal is performed, it is generally not possible to preserve nerve fibres, and reconstruction is required. Twenty-one patients in our series underwent total removal of tumour. Twenty of these 21 patients suffered a complete disruption to the nerve. In one case, however, the tumour was removed from the nerve, leaving the nerve completely intact and functioning normally (i.e. House–Brackmann grade I).
Nerve reconstruction is almost always required in cases of total tumour removal. Even so, O'Donoghue et al. were able to preserve at least 50 per cent of the facial nerve in 25 per cent of patients,Reference O'Donoghue, Brackmann, House and Jackler4 and McMenomey et al. achieved nerve preservation in 42 per cent of their patients.Reference McMenomey, Glasscock, Minor, Jackson and Strasnick15 This contrasts with our own experiences and those of other authors;Reference Kertesz, Shelton, Wiggins, Salzman, Glastonbury and Harnsberger3, Reference Liu and Fagan20, Reference Pulec22 in the present series, tumour removal from an intact facial nerve was possible in only one of 21 cases of total tumour removal.
Rarely, if the nerve gap is small, primary anastomosis may be performed. A short proximal stump poses significant technical difficulty for suturing within the depths of the cerebellopontine angle, but tissue glues may be used to approximate the nerve stumps. If the gap is too great for apposition without tension, the facial nerve may be rerouted by dividing the greater superficial petrosal nerve as well as freeing the tympanic and mastoid segments to mobilise the stumps, and then performing primary anastomosis. This manoeuvre requires division of the anterior tympanic artery at the geniculate ganglion, leading to a degree of devascularisation and poorer facial nerve outcomes. If the gap is too great for primary closure, then a nerve cable graft may be sutured between the nerve stumps, using sural or greater auricular nerve segments; there is no difference in outcomes for either donor nerve. A tensionless nerve repair is vital.
When the proximal nerve stump is too close to the brainstem to allow suturing, then a hypoglossal–facial anastomosis can be used. There are several variations on this theme. The hypoglossal nerve can be completely divided and the proximal hypoglossal nerve stump sutured end-to-end to the distal facial nerve stump. Alternatively, the hypoglossal nerve can be spilt and part of the proximal end sutured to the distal facial nerve stump. Another method entails removal of an epineural window from the hypoglossal nerve and suturing the distal facial nerve stump end-to-side to the otherwise intact hypoglossal nerve. A jump graft can also be used from the intact hypoglossal nerve to the distal facial nerve stump, if the distance is too great for direct suturing. Finally, the descendens hypoglossi branch of the hypoglossal nerve can be sectioned distally and swung superiorly to be sutured end-to-end to the distal facial nerve stump, with no discernable loss of function to swallowing from denervation of strap muscles.
Alternatively, a crossover facio–facial nerve graft can be performed. In this case, a long nerve graft (generally sural) is sutured to a redundant buccal branch of the intact contralateral facial nerve, tunnelled subcutaneously along the upper lip to the opposite side of the face and sutured to the ipsilateral nerve trunk.
Results of total tumour removal with primary anastomosis or nerve grafting generally show House–Brackmann grade III to IV recovery as the best possible outcome. Falcioni et al., in their series of nerve grafts, achieved 40 per cent grade III and 50 per cent grade IV recovery when the duration of deficit was less than one year.Reference Falcioni, Russo, Taibah and Sanna1 Our series had mean post-operative House–Brackmann grades of 3.0 for end–end anastomosis and 3.69 for nerve grafts.
Following hypoglosso–facial anastomosis, we achieved a mean post-operative House–Brackmann grade of 4.6 in five patients with end–end anastomoses, and a grade of 4.0 in one patient with a jump graft. Results from the literature suggest that House–Brackmann grades of III to IV can be expected. All six of the hypoglosso–facial anastomoses in our series were performed some time after tumour removal. They represented in essence a ‘back up’ plan used when other methods had failed, and hence were performed six to 12 months after the primary surgery. It is generally accepted that a short time interval is related to a better outcome.Reference Malik, Kelly, Ahmed, Saeed and Ramsden23 In their series, O'Donoghue and colleagues found no outcome advantage for any of the different reconstruction techniques used.Reference O'Donoghue, Brackmann, House and Jackler4
Facial rehabilitation
After facial nerve reconstruction, rehabilitation is important in order to optimise the level of recovery. Structured regimens of physiotherapy using graded exercise regimens, techniques to minimise synkinesis, and video self-modelling may improve results in motivated patients.
Gold weight insertion into the upper lid can be a temporary or permanent means of improving eye closure and preventing exposure keratitis and corneal ulceration. Lateral tarsorraphy is a more permanent method of improving eye closure, but can be reversed if unexpected recovery occurs. There are a range of facial reanimation procedures, using static or dynamic muscle and tendon slings to improve facial tone and movement. Orthograde temporalis transfer is a valuable dynamic reanimation in patients who do not wish to have a nerve substitution procedure, when the proximal facial nerve is unavailable for anastomosis. Static ‘fine tuning’ procedures such as brow lifting, unilateral facelift and blepharoplasty are used for improved cosmesis. Botulinum toxin is valuable for hyperkinesis and synkinesis following successful neural repair.
Conclusions
Facial schwannomas are rare lesions. With the almost universal availability of modern imaging techniques and a high index of clinical suspicion, the diagnosis of facial schwannoma should now generally be made pre-operatively, rather than unexpectedly at the time of surgery for presumed vestibular schwannoma, unless the tumour involves only the cerebellopontine angle and/or intracanalicular segments. Due to their complex management issues, facial schwannomas are best managed in a tertiary referral setting.
Observation is preferred until facial function deteriorates to a House–Brackmann grade III level, at which stage surgery must be considered; once facial function deteriorates to House–Brackmann grade IV, surgical intervention is strongly indicated. We advocate conservative surgical techniques such as drainage of cystic components, decompression and subtotal removal, in order to preserve native facial function as long as possible. When more aggressive measures are required, nerve preservation is almost never possible (we achieved this in one out of 21 cases of total tumour removal), and nerve reconstruction is required. An end-to-end anastomosis is the preferred method if the ends can be apposed without tension, but this is frequently impossible. A short proximal stump poses significant technical problems for suturing, but tissue glues may be used to approximate the nerve stumps. Rerouting of the facial nerve may gain some extra length, but this manoeuvre will also contribute to poor facial nerve function. The nerve gap may be bridged by a sural or greater auricular nerve graft. If the proximal nerve stump is too close to the brainstem for suturing, a hypoglosso–facial anastomosis should be performed. Adjunctive procedures, such as gold weight insertion, lateral tarsorraphy, or static or dynamic reanimation procedures, may also be required.

Fig. 6 H+E sections of Antoni A and B; IHC section; axons through lesion.

Fig. 7 CT scan: axial view, tumour involving tympanic segment.

Fig. 8 CT scan: coronal view, tumour involving labyrinthine and tympanic segments.

Fig. 9 MRI scan T1+gadolinium axial view: tumour extending from CPA to tympanic segment.

Fig. 10 MRI scan T1+gadolinium coronal view: tumour extending into middle fossa.

























