Introduction
Since its introduction a decade ago, transoral robotic surgery has gained increasing popularity in the management of oropharyngeal and other upper aerodigestive tract malignancies. Surgery with or without adjuvant therapy has shown promising results in the treatment of primary and salvage oropharyngeal malignancies.Reference Moore, Van Abel, Price, Lohse, Olsen and Jackson1–Reference Meulemans, Vanclooster, Vauterin, D'heygere, Nuyts and Clement4
Following transoral robotic surgery for primary malignancies, most resection sites are closed primarily or allowed to heal by secondary intention, without resulting in significant speech or swallowing deficits.Reference Ghanem5,Reference Genden, Kotz, Tong, Smith, Sikora and Teng6 This process of healing is often impaired in salvage surgical procedures because of previous radiotherapy, and often results in significantly longer healing times compared to primary cases.Reference Tomifuji, Araki, Yamashita and Shiotani7,Reference Jacobson, Johnson, Dedhia, Niknam-Bienia and Wong8 This leads to complications secondary to chronic non-healing wounds, such as infection, necrosis, the exposure of critical underlying structures and pain, causing increased patient morbidity.Reference Jacobson, Johnson, Dedhia, Niknam-Bienia and Wong8,Reference Dormand, Banwell and Goodacre9
Cases of complications secondary to failed healing of the surgical bed in salvage oropharyngeal surgery have been described.Reference Kao and Ooi10 Our department experienced two cases of necrotic ulceration within the lateral pharyngeal wall following transoral salvage surgery where reconstruction was not performed, resulting in pain and chronic infections. Meulemans et al. described late complications following salvage transoral robotic surgery resections. These included: necrosis over the resection site leading to spondylitis or spondylodiscitis 4 months following surgery; skull base osteomyelitis 2 years following transoral robotic surgery oropharyngectomy; and two cases of necrosis over the resection site, with one resulting in a carotid blowout and death 11 months following transoral robotic surgery resection.Reference Meulemans, Vanclooster, Vauterin, D'heygere, Nuyts and Clement4
Given these described complications, it is necessary to consider methods of intra-oral reconstruction that would complement the minimally invasive resection technique provided through transoral robotic surgery, with an aim to introduce healthy vascularised tissue to a previously irradiated field to aid healing and minimise further complications. Such reconstruction methods are often planned to: allow partial superior closure of larger transoral robotic surgery oropharyngeal resections; reduce soft palate defects to minimise speech and swallowing dysfunction secondary to velopharyngeal incompetence; provide soft tissue coverage of the carotid artery within the lateral oropharyngeal wall to prevent carotid blowout; and place vascularised tissue over exposed bone at the retromolar trigone to prevent further complications.Reference de Almeida, Park, Villanueva, Miles, Teng and Genden11,Reference Ayad, Kolb, de Mones, Mamelle and Temam12
There are limited studies currently available on salvage transoral robotic surgery for oropharyngeal malignancies; hence, there is a lack of data on suitable options for reconstruction in this setting. This paper describes the use of the facial artery musculomucosal flap as a method for lateral pharyngeal wall reconstruction following salvage transoral robotic surgery resection of oropharyngeal tumours.
Materials and methods
Institutional review board approval was obtained for a retrospective review covering the period of March 2015 to October 2018. All patients who were scheduled to undergo facial artery musculomucosal flap reconstruction following transoral robotic surgery resection of an oropharyngeal squamous cell carcinoma within a previously radiated field were included. These patients were selected because of the extent of resection required that would have otherwise left a large residual defect to heal by secondary intention or an exposed bony surface over the retromolar trigone, and/or would extend deep into the parapharyngeal space exposing neurovascular structures. In patients requiring neck dissection, this was performed in a staged fashion approximately one week prior to transoral robotic surgery.
General anaesthesia was performed, and the patient was intubated using a nasotracheal tube. The oropharyngeal area was exposed using a ‘FK-WO’ transoral robotic surgery retractor (Olympus, Hamburg, Germany) with the appropriate tongue blade. The da Vinci robotic surgical system (Intuitive Surgical, Sunnyvale, California, USA) was docked. The primary tumour was outlined with the appropriate margins and resection was performed. The resection margins were checked, and further margins were taken if there was a concern of close margins. Further tissue was taken from all margins for frozen section analysis. Once the surgical margins were confirmed as negative, the robot was de-docked. The reconstruction section of the procedure was then performed transorally via a standard surgical approach.
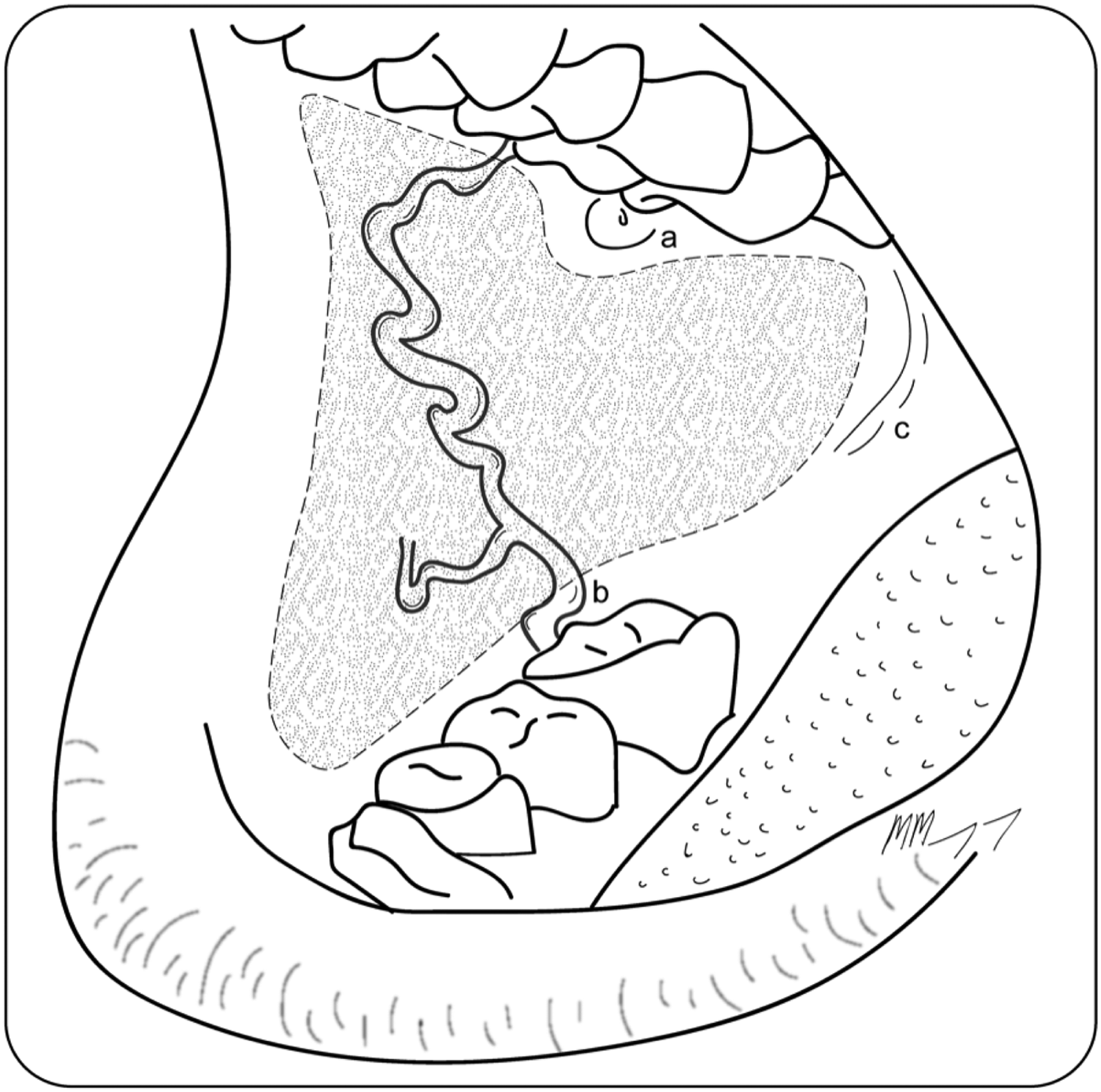
Primary closure velopharyngoplasty was performed in the superoposterior part of the oropharyngeal defect to limit velopharyngeal insufficiency; however, the facial artery musculomucosal flap often provided improved coverage and reconstruction. The buccal mucosa was outlined for harvest with identification and preservation of the parotid duct (Figure 1). The inferior incision extended from the retromolar trigone, across the inferior buccal mucosa anteriorly to 10 mm short of the lip. At least 10 mm of gingivobuccal mucosa was preserved inferiorly to prevent excessive wound contracture. The superior incision extended from the maxillary tuberosity, inferiorly to the parotid duct, and turned superiorly to capture a portion of upper lip mucosa. Anteriorly, approximately 10 mm of mucosa was preserved adjacent to the lip. Care was taken not to skeletonise the facial artery pedicle as, when islanded, venous drainage from the flap was reliant on the paired venae comitantes.

Fig. 1. Region of the facial artery musculomucosal flap within the buccal mucosa, with the parotid duct preserved superiorly (indicated by ‘a’), the course of the facial artery that supplies the flap running anterosuperiorly (represented by line ‘b’), and the inferior incision running from the retromolar trigone (indicated by ‘c’), anteriorly to 10 mm short of the lip.
The mucosa and the underlying buccinator muscle, which was pedicled inferiorly on the facial artery, was elevated and rotated into the lateral pharyngeal wall defect, taking care to avoid dental trauma to the pedicle (Figure 2). Incisions extended to the oropharyngeal defect to island the flap on the artery, allowing for greater manoeuvrability. In some cases, depending on the degree of rotation required, a posterior mucosal or buccinator bridge was preserved to improve venous outflow.

Fig. 2. Utilisation of the facial artery musculomucosal flap in lateral pharyngeal wall reconstruction. (a) Outline of a right facial artery musculomucosal flap and expected rotation (arrow) into a defect within the right lateral pharyngeal wall. (b) The inferiorly pedicled facial artery musculomucosal flap shown raised; the defect within the lateral pharyngeal wall is seen posteriorly. (c) The facial artery musculomucosal flap shown rotated to cover the lateral pharyngeal wall defect. The facial artery is seen lying posterior to the mandibular molar.
The flap was used to cover the lateral pharyngeal wall defect, reconstruct the soft palate, and cover critical structures such as an exposed artery or bone. Base of tongue defects were only partially covered because of the anatomical limits of the facial artery musculomucosal flap. An inferiorly pedicled flap was selected because of the improved arch of rotation to cover the regions mentioned above. Superiorly pedicled flaps cannot be rotated into the oropharynx.
The flap was sutured in place with size 4.0 PDS® (polydioxanone) sutures and size 3.0 Vicryl sutures. Small donor site defects were closed primarily. In larger donor site defects where the risk of oral contracture was deemed to be high, buccal fat was advanced into the defect; this was subsequently partially closed with size 3.0 Vicryl® and size 4.0 Vicryl Rapide sutures, or left to heal by secondary intention. Haemostasis was checked and confirmed. A nasogastric tube was inserted and secured.
Patients were treated with intravenous antimicrobials for 5 days. They were also exclusively fed through a nasogastric tube for a minimum of 5 days, to allow for sufficient flap healing and to minimise wound breakdown, whilst maintaining adequate nutrition. Patients were taught to perform gentle cheek massages to minimise swelling and pooling of saliva within the donor site. Early speech pathology input was utilised in all patients, providing guidance and supervision for the safe introduction of oral intake and education on mouth exercises to minimise oral contracture and trismus.
Specimen and tumour size details were obtained from the final histology reports. Length of stay, surgical complications, and speech and swallow outcomes were recorded. The radiation dosage received by patients to the region of the facial artery musculomucosal flap during previous treatment was retrospectively reviewed for the purposes of this study. Contouring of the buccal donor site region was performed using the consensus guidelines for computed tomography based delineation of organs at risk in the head and neck region.Reference Brouwer, Steenbakkers, Bourhis, Budach, Grau and Gregoire13
Results
Thirteen patients underwent facial artery musculomucosal reconstruction following salvage transoral robotic surgery for oropharyngeal squamous cell carcinoma, all within a previously radiated field. The procedures were performed at the Peter MacCallum Cancer Centre (Melbourne) and at Epworth Healthcare (Richmond) in Australia.
Median patient age was 61 years (interquartile range, 59–63 years). Nine patients were male. Eight patients presented with a new primary tumour within a previously irradiated field, three presented with tumour recurrence and two presented with a residual tumour following previous chemoradiotherapy. The tumour subsites were as follows: six base of tongue cases, six tonsil cases and one posterior pharyngeal wall case. Pathological tumour (T) stage, determined using the eighth edition of the American Joint Committee on Cancer's AJCC Cancer Staging Manual,Reference Amin, Edge, Greene, Byrd, Brookland and Washington14 was T1 in five patients, T2 in seven patients and T4 in one patient.
Previous radiotherapy had been received by eight patients to the ipsilateral oropharynx and neck, by three patients to the contralateral oropharynx and neck, by one patient to the ipsilateral oral cavity and neck, and by one patient to the ipsilateral hypopharynx and neck. Information regarding radiotherapy treatment fields was available for four patients (31 per cent). Patients received a mean dose of 37 Gy (range, 6.5–53.8 Gy) and a maximum dose of 58 Gy (range, 20.9–75.0 Gy) to the region of the facial artery musculomucosal donor sites.
Two patients had undergone ipsilateral neck dissections during their previous treatment. Three patients underwent staged neck dissections a week prior to transoral robotic surgery, which included ligation of the ipsilateral lingual artery. Two were bilateral neck dissections and one was a unilateral neck dissection. There were no complications associated with the neck dissections in this study.
Two patients underwent planned tracheostomy insertion at the time of transoral robotic surgery resection. One tracheostomy was performed because the resection involved a supraglottic laryngectomy. The second patient had previously been treated with contralateral oral and base of tongue resections, free flap reconstruction, and adjuvant radiotherapy. A planned tracheostomy was performed as the residual tongue function following the salvage transoral robotic surgery base of tongue resection was predicted to be poor. Both patients were successfully decannulated prior to discharge home.
Median length of stay in hospital was 9 days (interquartile range, 9–13 days). There were no cases of flap breakdown, partial flap failure or flap loss in this series. There was one in-patient complication requiring surgical management (7.6 per cent): bleeding from the facial artery musculomucosal donor site required surgical haemostasis under general anaesthetic. There was one minor complication: a case of infection at the donor site, which was treated with antimicrobials and regular oral flushing. One patient required division of the facial artery musculomucosal pedicle after discharge from hospital, three weeks following surgery, as the facial artery pedicle of the flap was running over the adjacent molar tooth during oral intake.
Eight patients required a nasogastric or gastrostomy tube on discharge, four for supplemental nutrition and four for severe dysphagia. One of these patients required a gastrostomy tube prior to surgery for dysphagia secondary to recent chemoradiotherapy. At the six-month mark, four patients (31 per cent) remained completely nil orally and gastrostomy tube dependent for severe dysphagia.
Initial speech assessment revealed mild dysarthria in six patients (46 per cent), moderate dysarthria in five patients (38 per cent) and severe dysarthria in two patients (15 per cent). Assessment details were not available for one patient. The majority of patients remained more than 90 per cent intelligible during communication (n = 12, 92 per cent). There were no reported cases of velopharyngeal insufficiency. At the six-month mark, eight patients (62 per cent) were assessed as having mild to no dysarthria, two patients (15 per cent) had moderate dysarthria, and one patient (8 per cent) had severe dysarthria. Information was not available for two patients.
Median follow-up time in this study was 15 months (interquartile range, 5.0–26.0 months). There were no late complications in these patients, with no episodes of delayed healing or necrosis.
Discussion
Previous studies have investigated reconstructive options following primary transoral robotic surgery for oropharyngeal malignancies, utilising local reconstructive options such as unilateral or bilateral facial artery musculomucosal flaps, a posteromedially based musculomucosal flap, a nasoseptal flap, and transposition flaps such as the temporalis muscle flap.Reference de Almeida, Park, Villanueva, Miles, Teng and Genden11,Reference Bonawitz and Duvvuri15–Reference Meccariello, Montevecchi, Deganello, D'Agostino, Bellini and Zeccardo17 Several series have described robotic-assisted free tissue transfers utilising the radial forearm, and vastus lateralis and anterolateral thigh free flaps for larger defects, with good wound and functional results.Reference Ghanem5,Reference Genden, Kotz, Tong, Smith, Sikora and Teng6,Reference Mukhija, Sung, Desai, Wanna and Genden18–Reference Biron, O'Connell, Barber, Clark, Andrews and Jeffery22
Several studies have reported improved functional and survival outcomes following salvage transoral robotic surgery; however, no reconstruction was described in the majority of these studies.Reference White, Ford, Bush, Holsinger, Moore and Ghanem3,Reference Meulemans, Vanclooster, Vauterin, D'heygere, Nuyts and Clement4,Reference Dabas, Dewan, Rangan, Dewan, Shukla and Sinha23–Reference Al-Khudari, Bendix, Lindholm, Simemrman, Hall and Ghanem25 The general aims of reconstruction in salvage cases are to provide healthy vascularised tissue to a previously radiated field, to improve wound healing and to provide stability to the repair.Reference Jacobson, Johnson, Dedhia, Niknam-Bienia and Wong8 Local flaps have generally been avoided as reconstructive options in salvage cases, because of the risk of radiation-induced vascular injury to the donor site, and the subsequent potential increased risk of wound complications and flap failure.Reference Selber, Sarhane, Ibrahim and Holsinger20
The facial artery musculomucosal flap was first described by Pribaz et al. in 1992.Reference Pribaz, Stephens, Crespo and Gifford26 It acts as a good local reconstruction option, where the harvest and inset of the flap is easily performed transorally, either with a headlight as performed in this series, or robotically as described in Bonawitz and Duvvuri.Reference Bonawitz and Duvvuri15 Utilising a facial artery musculomucosal flap avoids the need for external scars over the neck and the creation of a communication between the oropharynx and neck, as would be required for free flap micro-anastomosis. The facial artery musculomucosal flap has a large axis of rotation and range, allowing it to reach areas within the oropharynx.Reference Pribaz, Stephens, Crespo and Gifford26,Reference Ayad and Xie27 It is a thin and pliable flap composed of fully functional mucosal tissue, minimising excessive bulk that may be seen with free flap alternatives.Reference Berania, Lavigne, Rahal and Ayad28
When utilised for reconstruction following transoral robotic surgery resection for primary oropharyngeal malignancy, the facial artery musculomucosal flap showed minor wound complications, with good speech and swallow outcomes.Reference Bonawitz and Duvvuri15 A systematic review on facial artery musculomucosal flaps utilised for head and neck reconstructions revealed an overall 12.2 per cent partial necrosis rate, a 2.9 per cent complete necrosis rate, and a 12.8 per cent combined minor complication rate (which included wound dehiscence, venous congestion, haematoma and infection).Reference Ayad and Xie27
Individual studies have shown varying results following the use of facial artery musculomucosal flaps in previously radiated patients, largely for floor of mouth reconstruction.Reference Ayad, Kolb, de Mones, Mamelle and Temam12,Reference O'Leary and Bundgaard29 In both these studies, the radiation doses previously received by the donor site were not described.
In our series of 13 salvage patients, there were no cases of wound dehiscence, partial flap failure or flap loss. Based on the contouring performed on four patients where radiation treatment field data were available, our patients received a mean dose of 37 Gy and a maximum dose of 58 Gy to the donor sites. Whilst it may be difficult to determine the exact radiation dose received at the donor sites for all salvage patients, and to determine the relationship between dose and flap-related complications, this may be an interesting area for future research. This knowledge may guide surgeons in predicting which patients may be at risk of facial artery musculomucosal flap complications following its utilisation in salvage reconstruction.
The facial artery musculomucosal donor site can often be closed primarily if the defect is smaller than 3 cm wide, it can be left to heal with secondary intention, or it can be closed with a buccal fat pad advancement to prevent donor site contracture and trismus.Reference Ayad and Xie27 We utilised a combination of these methods based on individual defect characteristics and surgeons’ discretion. None of the 13 patients in this series had a significant contracture that impaired swallow or speech efforts, and which would have required surgical release. It is important to assess each case individually when performing donor site closure, to minimise donor site complications.
• Facial artery musculomucosal flaps are commonly used for oral and oropharyngeal reconstruction in primary resection cases
• Studies reporting the use of facial artery musculomucosal flaps for oral reconstruction in salvage situations have shown variable flap and donor site complications
• There were no partial or full facial artery musculomucosal flap failures when utilised for lateral pharyngeal wall reconstruction following salvage transoral robotic surgery in our series
• Facial artery musculomucosal flaps demonstrated minimal donor site morbidity in our patients
In this series, there was a 31 per cent long-term gastrostomy tube requirement as a result of severe dysphagia and high aspiration risk. This is thought to be due to the extent of surgical resection and the results of previous radiation treatment, not due to flap bulk or velopharyngeal insufficiency. Our impression is that this rate would not have been reduced using a different reconstructive method.
Limitations
There is a selection bias in this study, where only patients thought to be successful candidates for a facial artery musculomucosal flap were offered this reconstructive option. Not all patients may be suitable for facial artery musculomucosal reconstruction following salvage transoral robotic surgery for oropharyngeal malignancies, and each case should be evaluated on an individual basis. However, this study provides the largest series of patients who underwent facial artery musculomucosal reconstruction of lateral oropharyngeal wall following salvage transoral robotic surgery, and the results suggest good outcomes with limited patient morbidity.
Conclusion
The facial artery musculomucosal flap provides a good option for local flap reconstruction of the lateral pharyngeal wall in selected patients undergoing salvage transoral robotic surgery for oropharyngeal malignancies. It allows for healthy, vascularised tissue to be introduced to the resection bed, to aid healing and minimise potential complications associated with poor wound healing.
Competing interests
None declared