Introduction
Developing skills in laryngeal microsurgery poses a special challenge in resident training. While most programmes provide adequate experience in diagnostic laryngoscopy, few offer adequate training in phonosurgery. Many of these procedures are performed upon professional voice users and allow little tolerance for a beginner's learning curve.
Simulation has gained wide acceptance in medical student and resident education; it provides trainees with first-hand exposure to emergency situations and complex procedures, while minimising risk to patients. This is particularly true for surgical training, where simulation allows students to acquire mechanical skills and enables teachers to document technical competence in a standardised fashion.Reference Aggarwal and Darzi 1
Several otolaryngologists have proposed experimental models for teaching phonosurgery skills.Reference Holliday, Bones, Malekzadeh and Grant 2 , Reference Conroy, Surender, Geng, Chen, Dailey and Jiang 3 These generally take advantage of excised humanReference Amin, Rosen, Simpson and Postma 4 or animal laryngesReference Nasser, Wahba, Kamal, El-Makhzangy and Bahaa 5 mounted in custom holders.Reference Dailey, Kobler and Zeitels 6 While of proven value, these holders are expensive to build and are not widely available.Reference Dedmon, Paddle, Phillips, Kobayashi, Franco and Song 7 They accurately reproduce the experience of laryngeal microdissection, but do not teach the skills required for suspension laryngoscopy.Reference Tan, Prufer, Chinosornvatana, Park and Woo 8
Our group recently described the use of a fresh, saline-perfused sheep head and neck model for surgical simulation.Reference Isaacson, Ianacone and Wolfson 9 This article explores its use for suspension microlaryngoscopy and phonosurgical procedures.
Materials and methods
Tissue was collected from pre-pubescent sheep (n = 10; mean age of 4 months, with a mean mass of 28 kg) following humane euthanasia (100 mg/kg sodium pentobarbital solution) at the end of an in vivo protocol approved by the Temple University Lewis Katz School of Medicine through its Institutional Animal Care and Use Committee. These sheep were obtained from a Temple University and US Department of Agriculture approved supplier, tested for Coxiella burnetii according to Centers for Disease Control and Prevention protocol, and accepted into the University Laboratory Animal Resource facility of Temple University after full inspection by a licensed veterinarian to assure good health.
No live animals were used in this study. Post-euthanasia, the head and neck of the sheep were saline-perfused and disarticulated 4–6 cm above the sternal notch. The tissues were stored at 5 °C for 1–5 days.
Prior to working with fresh ovine tissue, study personnel were enrolled in the Temple University's occupational health programme and screened for query fever (‘Q fever’) antibodies. They wore appropriate personal protective clothing during experimentation. Sheep tissues were transported in double plastic disposable bags and all carcass material was treated as biomedical waste through a commercial waste management company.
The ovine head and neck preparation was supported in a supine position with sand bags and adhesive tape passed around the operating table (Figure 1). This provided laryngeal counter-traction against the suspension apparatus.

Fig. 1 Ovine head and neck preparation supported in supine position with sand bags.
Karl Storz adolescent Parsons and adult Kantor-Berci laryngoscopes (Tuttlingen, Germany) were used for exposure. They were supported by a Benjamin-Parsons laryngoscope holder resting on a cardboard box. A 4 mm, 0° or 30° Karl Storz rigid rod endoscope provided illumination and magnification when using the Parsons laryngoscope. The Kantor-Berci laryngoscope has its own integrated rigid endoscope. A Karl Storz Tele Pack X mobile endoscopic video system was placed at the foot of the operating table for image visualisation. It features a light-emitting diode (‘LED’) light source and has an integrated video recording system.
A Karl Storz Shapshay-Ossoff microlaryngeal instrument set was used for endolaryngeal dissection. This included rigid laryngeal microsuction tubes. A 3 ml syringe filled with saline and a 22 gauge, 7 inch (17.78 cm) spinal needle were available for simulation of injection laryngoplasty.
Procedures were performed in three half-day sessions by a fellowship-trained laryngologist and a paediatric otolaryngologist working with a second year medical student. The student had the opportunity to practise micro-suspension, visualisation, injection and operative procedures under the guidance of his mentors.
Results
The model proved highly realistic for suspension microlaryngoscopy. The fresh sheep have relatively strong rigor mortis in the first 48 hours, so it is best to use the preparation on the 3rd day or later after sacrifice. Despite proportional differences in the jaws and larynx, standard human suspension laryngoscopes worked well in the young sheep model (Figure 2). The long, high-riding sheep epiglottis is best handled by placing the laryngoscope on the laryngeal surface of the epiglottis, rather than using it as a vallecular blade. The sheep lacks easily identifiable false vocal folds and ventricles. The arytenoids are prominent, and occupy more of the laryngeal introitus than is seen in humans, dogs or pigs. This made for easy exposure of the arytenoids, but limited access to the vocal folds with the Parsons laryngoscope (Figure 3). The Kantor-Berci laryngoscope provided better anterior exposure and allowed for surgical manipulation of the membranous vocal folds. The use of laryngeal counter-pressure improved visualisation, as did the use of a 30° telescope. With the head and neck preparation in suspension, injection laryngoplasty (Figure 4), hydrodissection and incision, endolaryngeal suturing (Figure 5), and partial cordectomy were all practical. The model is appropriate for endolaryngeal arytenoid surgical procedures, though these were not performed in this study.
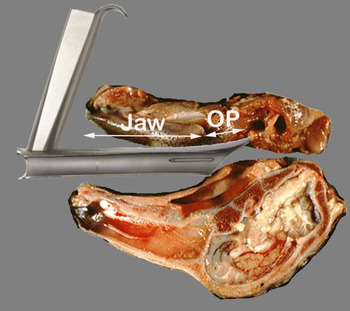
Fig. 2 Illustration of the Parsons laryngoscope in suspension – sagittal section. Despite differences in anatomy, ovine laryngeal exposure is possible. OP = oropharynx

Fig. 3 Endoscopic view of ovine glottis through an adolescent Parsons laryngoscope. v = membranous vocal fold; a = arytenoid

Fig. 4 Endoscopic view of right vocal fold injection through a Kantor-Berci laryngoscope.

Fig. 5 Endoscopic view of endolaryngeal suturing through a Kantor-Berci laryngoscope.
Discussion
An ideal model for surgical simulation in endoscopy training should: (1) be readily available to most training programmes; (2) be inexpensive; (3) have a low risk of disease transmission; and (4) accurately reflect normal human anatomy.
Several authors have described using excised larynx models for learning microlaryngoscopy skills.Reference Amin, Rosen, Simpson and Postma 4 – Reference Tan, Prufer, Chinosornvatana, Park and Woo 8 , Reference Awad, Patel, Hayden, Sandhu and Tolley 10 Models employing fresh human larynges have an anatomical accuracy that no animal model can equal.Reference Howard, Mendelsohn and Berke 11 Unfortunately, fresh human tissues are increasingly difficult to obtain and have the highest risk of disease transmission of any model.Reference Shoja, Benninger, Agutter, Loukas and Tubbs 12 The authors have worked with in vivo and ex vivo human, canine and porcine models during surgical research,Reference Isaacson 13 and during courses for the instruction of residents and fellows.Reference Deutsch, Christenson, Curry, Hossain, Zur and Jacobs 14 The dog model is among the best for simulation of laryngeal anatomy and physiology.Reference Howard, Mendelsohn and Berke 11 Animal rights concerns have greatly complicated the use of canine specimens in research.Reference Hasiwa, Bailey, Clausing, Daneshian, Eileraas and Farkas 15 Young pigs are frequently used for simulation. Pigs grow very rapidly and become unworkably large after two to three months of age. Their long snouts and jagged teeth limit the use of conventional human suspension laryngoscopes.
The fresh ovine head and neck model accommodates conventional suspension laryngoscopy equipment and does not require a custom holder for the larynx. The sheep mandible and maxilla are proportionally longer than human jaws. At the same time, the distance from the oropharynx to the larynx is shorter. The net result is an upper airway that easily accommodates standard equipment designed for human surgery.
While the structure of the sheep larynx varies from human anatomy, it is sufficiently analogous to allow the most common of surgical procedures, including injection laryngoplasty, vocal fold lesion excision, hydrodissection and incision, and micro-suturing. Our experience was similar to that of Tan et al.Reference Tan, Prufer, Chinosornvatana, Park and Woo 8 They suspended a freshly excised lamb's larynx, and operated using a microscope and the relatively large RealHand™ instruments. They found the proportionately short membranous vocal folds challenging for micro-trapdoor dissection, and the large arytenoids were less than ideal for endoscopic arytenoidectomy. In contrast, we found the relatively large size of the arytenoids facilitated visualisation in the surgical field. Anterior commissure laryngoscopes such as the Kantor-Berci allowed best exposure of the vocal folds and permitted easier dissection. We chose to use endoscopic visualisation in these experiments. With proper set-up, the same ovine head and neck preparation could be used for: endolaryngeal dissection with an operating microscope, excision with various lasers, or robotic laryngopharyngeal surgery.
Sheep are widely available in the USA and around the world. We were fortunate to have access to a regular supply of sheep head and neck preparations through a shared tissue programme at Temple University. For those who do not have such access, it is possible to use farm-raised animals for the same purpose.Reference Gardiner, Oluwole, Tan and White 16 This would require a relationship with an abattoir, as most sheep carcasses delivered to meat markets have been eviscerated. At approximately $4 per pound, each head and neck preparation would cost $20–$28.
A discussion of zoonotic disease is relevant when considering the handling of fresh ovine tissue. Sheep rarely transmit infections to humans.Reference Huijskens, Smit, Rossen, Heederik and Koopmans 17 The main concern with using mature farm animals is query fever, a treatable but potentially dangerous disease. The causative bacteria, C burnetii, are found primarily in pregnant sheep, residing in the amniotic fluid and placental tissue. The major route for human contamination is aerosolised dried amniotic and placental material in barnyard dust. 18 Should a programme choose to use farm-raised animals, selecting tissues from male or pre-pubescent female sheep should reduce the risk for C burnetii exposure.Reference Whitney, Massung, Kersh, Fitzpatrick, Mook and Taylor 19
-
• Despite variations in proportion and structure, the sheep head and neck preparation provides an inexpensive, safe model
-
• Such a model can be used to develop suspension laryngoscopy and basic phonosurgical skills
-
• Use of an intact head and neck preparation obviates the need for expensive, custom-made tissue holders
-
• Standard adult laryngoscopes and phonosurgical instruments can be used
-
• Risks of zoonotic infection are low, and should be negligible if male or pre-pubescent female sheep are used
-
• The model is appropriate for endolaryngeal surgery with an operating microscope and lasers delivered by micromanipulator or flexible fibres
Several others have demonstrated with utility of animal models for teaching large groups.Reference Dedmon, Paddle, Phillips, Kobayashi, Franco and Song 7 We did not attempt duplication of this work, but plan to use the model for group teaching as part of our resident training programme.
Acknowledgement
Supported in part by Department of Defense/Office of Naval Research grants N000141210810 and N000141210597.







