Introduction
Epistaxis is a common emergency presentation to both primary and secondary care. It is well established that anticoagulation and hypertension increase the risk of epistaxis.Reference Smith, Siddiq, Dyer, Rainsbury and Kim1–3 However, it is unclear whether other non-anticoagulant medications are associated with epistaxis.
A clinic patient attending with epistaxis drew our attention to the product literature stating that epistaxis is a side effect of taking atorvastatin. The British National Formulary lists epistaxis as a ‘common or very common’ side effect of atorvastatin, unlike simvastatin and pravastatin which have no risk of epistaxis.4 Interestingly, another ‘common or very common’ listed side effect of atorvastatin is nasopharyngitis, which may have a role in epistaxis.
The British National Formulary is likely acknowledging the side effects discovered in atorvastatin's clinical trials and post-marketing experience. The atorvastatin placebo-controlled clinical trial database and US Food and Drug Administration states that epistaxis was reported in less than 2 per cent of patients taking atorvastatin, regardless of causality.5,6 Nasopharyngitis was the most common side effect, occurring in 8.3 per cent of people (727 out of 8755) who took atorvastatin.5
Statins inhibit 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase to reduce cholesterol synthesis. Atorvastatin is a fully synthetic inhibitor, compared with simvastatin and pravastatin which have a decalin ring structure.Reference Istvan and Deisenhofer7 Statins have been shown to cause oral dryness and nasopharyngitis, and to increase the risk of haemorrhagic stroke, haematuria and ocular haemorrhage, which could explain the increased susceptibility to epistaxis.Reference Cruz, Kustner, Vicente, Ferrero, Thio and Lopez8 Statins have antithrombotic properties and increase the risk of thrombocytopaenia, which may explain many of these side effects.Reference Golomb and Evans9 However, it is unclear why atorvastatin is the only statin associated with epistaxis.
We aimed to investigate whether there was an association between atorvastatin and epistaxis requiring hospital admission. As we had not been aware of the British National Formulary literature in question, we also aimed to gauge awareness of it within the ENT specialty.
Materials and methods
Audit department approval to conduct the study was granted (number 61701). National Research Ethical Service approval was not required.
Data were collected retrospectively, using the 10th revision of the International Classification of Diseases (‘ICD-10’) code R04 to identify adult patients admitted with epistaxis. We selected 100 consecutive patients, from November 2016 to August 2017, with a median age of 71 years (range, 19–94 years). The mode of intervention, need for operative intervention, length of hospital stay and re-attendances to hospital were recorded. Data on statin, antiplatelet and anticoagulant medication use were collected from electronic in-patient records.
A two-question survey of ENT registrars aimed to identify awareness of the British National Formulary information listing epistaxis as a side effect of atorvastatin. A total of 24 ENT registrars (specialty trainees, years three to eight; ST3–ST8), responded on 18 December 2017.
Data were analysed using SPSS statistical software (version 21; IBM, Washington, DC, USA). Pearson's chi-square test was used to determine associations between categorical variables. Statistical significance was defined as p < 0.05.
Results
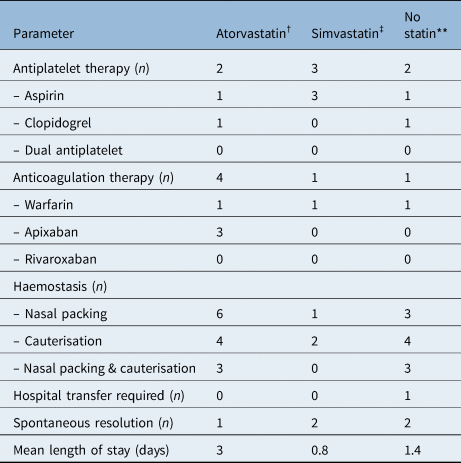
Of the 100 patients (53 males and 47 females) admitted with epistaxis, 27 were on atorvastatin, 21 were on simvastatin and 2 were on pravastatin. In total, 52 patients were receiving either an antiplatelet or anticoagulant, or both. The prevalence of patients taking antiplatelets was significantly higher in the atorvastatin group compared to the non-statin group (p = 0.0003). However, there was no significant difference between the number of patients taking anticoagulants in the atorvastatin group versus the non-statin group (p = 0.19). There was no significant difference in antiplatelet use between the atorvastatin group and simvastatin group (p = 0.08) (Table 1). The most common daily atorvastatin dose was 40 mg (n = 13), followed by 20 mg (n = 8), 80 mg (n = 5) and 10 mg (n = 1).
Table 1. Patients admitted with epistaxis by statin type*

* n = 100;
† n = 27;
‡ n = 21;
** n = 2;
§ n = 50.
SPA = sphenopalatine artery
The most frequent method of haemostasis was nasal packing in all groups. Two patients required sphenopalatine artery ligation and one patient received electrocautery under general anaesthesia (Table 1). One of the patients who underwent sphenopalatine artery ligation was receiving warfarin, and the patient who underwent electrocautery was taking clopidogrel. None of these three patients were receiving statin therapy.
Of the 100 patients admitted with epistaxis, 19 were readmitted with a further episode of epistaxis within the following year. Of these patients, seven were taking atorvastatin, five were taking simvastatin and seven were not taking statins. One patient required transfer to the regional head and neck centre to manage their epistaxis; this patient was not taking any statins, antiplatelets or anticoagulants (Table 2).
The questionnaire revealed that none of the 24 ENT registrars were aware that epistaxis was listed as a common side effect of atorvastatin in the British National Formulary. All 24 ENT registrars said they would consider asking the general practitioner to substitute atorvastatin for another statin in cases of recurrent epistaxis.
Discussion
Epistaxis is very common; its incidence is difficult to quantify because it is often self-limiting. The summary of product characteristics in the Electronic Medicines Compendium and the British National Formulary list epistaxis as a common side effect of atorvastatin.4,10 A common side effect is estimated to occur in 1–10 per cent of patients.10 The results of the phase 3 atorvastatin placebo-controlled trials and the yellow card reporting system will both have influenced atorvastatin's side effect profile in the Electronic Medicines Compendium and British National Formulary.10 The reported frequency of epistaxis in those taking atorvastatin (n = 8755) in placebo-controlled trials was less than 2 per cent, which is likely to be similar to the rate in the general population.5 A review of the five listed placebo-controlled trial manuscripts revealed no information relating to epistaxis.
Half of the patients in our population were receiving statin therapy when admitted with epistaxis. Only atorvastatin is associated with epistaxis in the British National Formulary. A similar number of patients were taking simvastatin, which has no such reported association in the British National Formulary.4
Antiplatelet and anticoagulant therapy is known to increase patients’ risk of epistaxis.Reference Smith, Siddiq, Dyer, Rainsbury and Kim1,3 We found no significant difference in concurrent antiplatelet or anticoagulant use in patients receiving atorvastatin and simvastatin. However, significantly more patients taking atorvastatin were also receiving antiplatelet therapy compared with patients not on statin therapy. This could partly explain why patients receiving atorvastatin may have a perceived higher risk of epistaxis compared to the non-statin-taking population.
• Epistaxis is listed as a common side effect of atorvastatin in the British National Formulary
• However, there is no available literature investigating the impact of statins on epistaxis
• In this study, half of the patients admitted with epistaxis were receiving statin therapy
• Patients taking atorvastatin were more likely to be receiving concurrent antiplatelet therapy than non-statin therapy patients
• Knowledge of the side effect profile of atorvastatin is frequently unknown
• There is no evidence that patients receiving atorvastatin have an increased risk of epistaxis compared with those taking simvastatin
None of the severe epistaxis patients requiring haemostasis in the operating theatre were taking statins. Two of three were receiving either anticoagulants or antiplatelets. In addition, the mean length of hospital stay was not affected by statin use (Table 1).
Although very limited evidence currently exists, knowledge of atorvastatin's listed side effects in the British National Formulary was non-existent in the sample cohort of ENT registrars.4 Clinicians have an obligation to be aware of this listed side effect. However, the current study found no evidence that patients receiving atorvastatin have a higher risk of epistaxis compared with those taking simvastatin, or that the use of any statin caused severe epistaxis necessitating surgical intervention. Thus, it is unlikely that ENT services are neglecting an important cause of severe epistaxis.
This study is limited in that only patients requiring admission for epistaxis were included. Epistaxis occurs most frequently in the community and only severe episodes of epistaxis require hospital admission.
There is currently no literature describing the alleged association between atorvastatin and epistaxis. In our study, which had a small sample size, there was no evidence that atorvastatin is associated with an increased risk of epistaxis requiring hospital admission, when compared to simvastatin. We suggest that the potential association between atorvastatin and epistaxis needs further investigation with larger multicentre studies, based in both primary and secondary care. The pharmaceutical literature should then be appropriately amended.
Competing interests
None declared