Introduction
Prosper Ménière presented a paper to the French Academy of Medicine in 1861 in which he described patients with hearing loss, episodic vertigo and non-pulsatile tinnitus.Reference Morrison1 This paper suggested that damage or disease in the inner ear, or more precisely the semicircular canals, was responsible for these symptoms, and not cerebral congestion as was the belief of the time.
In 1938, Cairns and Hallpike observed endolymphatic hydrops in temporal bone specimens from patients with Ménière's disease.Reference Cairns and Hallpike2 They described the inner ear as like a ‘balloon containing fluid’. They believed the tinnitus and vertigo were due to ischaemia of the end organs in the wall of the membranous labyrinth, a result of the pressure effects of hydrops.
In standard anatomical descriptions, the endolymphatic sac is part of the membranous labyrinth. A narrow tube joins the utricle and saccule, and forms the root of the endolymphatic duct. The duct passes through the bony aqueduct of the vestibule to end in a blind dilation which is the endolymphatic sac. This is often described in parts, an intraosseous (proximal and intermediate) part medial to the operculum, and an intradural (distal) part lying between the two layers of the dura in the endolymphatic fossa close to the sigmoid sinus.Reference Schindler3
Surgery performed on the endolymphatic duct or sac is carried out to decrease the pressure in the endolymphatic space, whilst preserving hearing and vestibular function. Portmann, in 1927, presented a paper on an operation performed to drain the saccus endolymphaticus for the relief of vertigo.Reference Portmann4 The operation involved decompressing the endolymphatic sac by removing the lateral bony wall and making a small incision into the lumen of the sac to allow the free drainage of endolymph.
Transmastoid decompression of the endolymphatic sac has always been a controversial procedure and has a chequered history. Thomsen and colleagues conducted a placebo-controlled study in 1981 in which patients underwent endolymphatic decompression or cortical mastoidectomy.Reference Thomsen, Bretlau, Tos and Johnsen5 They concluded that the effect of the surgery was not related specifically to the decompression but was in fact a placebo effect. However, in 1996 it was claimed that endolymphatic sac decompression was a good surgical first choice, with a low rate of complications.Reference Södermann, Ahlner, Bagger-Sjöbäck and Bergenius6 Ten years later, this surgery was reported as being likely to improve patient perception of symptoms and quality of life.Reference Durland, Pyle and Connor7, Reference Convert, Franco-Vidal, Jean-Pierre and Darrouzet8 By 2008, the procedure was well established, but it remained controversial, and investigators were less sanguine about the results.Reference Wetmore9 This ambiguity regarding the effectiveness of surgical intervention in the form of endolymphatic sac decompression persists, as revealed by a recent Cochrane review.Reference Pullens, Giard, Verschuur and van Benthem10
Our experience with cadaver dissection suggested that the results of surgical decompression may be unpredictable because the endolymphatic sac is difficult to locate and decompress adequately. Furthermore, prolonged exploratory dissection in the region of the sac is likely to increase the risk of complications as a result of damage to the sac and surrounding structures.
In order to test this hypothesis, five simulated decompressions were performed on cadaver heads via a transmastoid route. In each, an attempt was made to identify the endolymphatic sac and confirm its identity by histology. Fourteen half heads were dissected topographically to assess the size, shape and position of each sac. The presence of the sacs’ surface epithelium in the 14 cases was confirmed using scanning electron microscopy. A note was made of the structures at risk during dissection.
Materials and methods
All material was from the standard stock of embalmed cadavers at the Department of Anatomy, University of Glasgow. Cadavers were initially injected with embalming fluid through the right common carotid artery. Latex and Indian ink were then injected into the arterial system and allowed to set.
Transmastoid endolymphatic sac decompression was performed on five half heads. The dense bone surrounding the posterior semicircular canal was identified and thinned. Bone was then removed from the posterior cranial fossa dura between the posterior semicircular canal and the sigmoid sinus, and between the superior petrosal sinus and the jugular bulb. The sigmoid sinus needed compressing so the bone directly medial to it could be removed and the distal part of the sac identified. In order to demonstrate the proximity of the facial nerve to the sac, the vertical segment of the facial nerve was decompressed.Reference Locke11 An attempt was made to identify the entire sac by direct vision.
The dura of the posterior cranial fossa was then excised and processed for histology. The sections were stained with haematoxylin and eosin to allow examination of the endolymphatic sac within the dura, and to confirm the presence of the lumen from the intradural part of the sac.
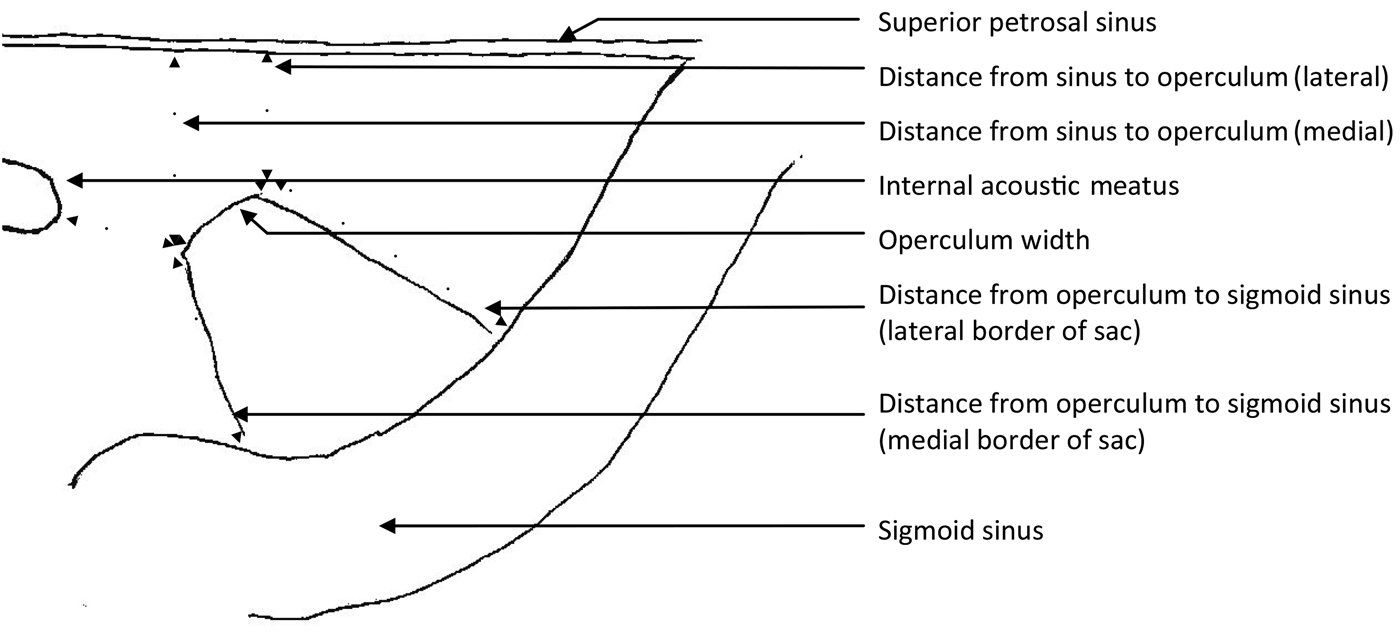
The posterior cranial fossa dura from a further 14 half heads (from 10 cadavers) was then dissected to assess the intradural component of the endolymphatic sac. From the posterior cranial fossa side, the layer of dura covering the endolymphatic fossa was peeled off from the operculum along the long axis of the sac to the sigmoid sinus, uncovering the sac. The wall of the sac was then excised and the surface epithelium confirmed by scanning electron microscopy. Measurements of the maximum width of the sac, and of the length along the medial and lateral borders were taken with callipers. The width of the operculum was measured. The distance from the operculum to the sigmoid sinus was measured along the medial and lateral borders of the sac. The distance from the medial and lateral limits of the operculum to the superior petrosal sinus, and the shortest distance to the internal acoustic meatus, were then measured (Figure 1). All measurements were taken twice by the same person on separate occasions and a mean of the two results was then recorded. From the measurements, standard deviations and the coefficient of (biological) variation were calculated.
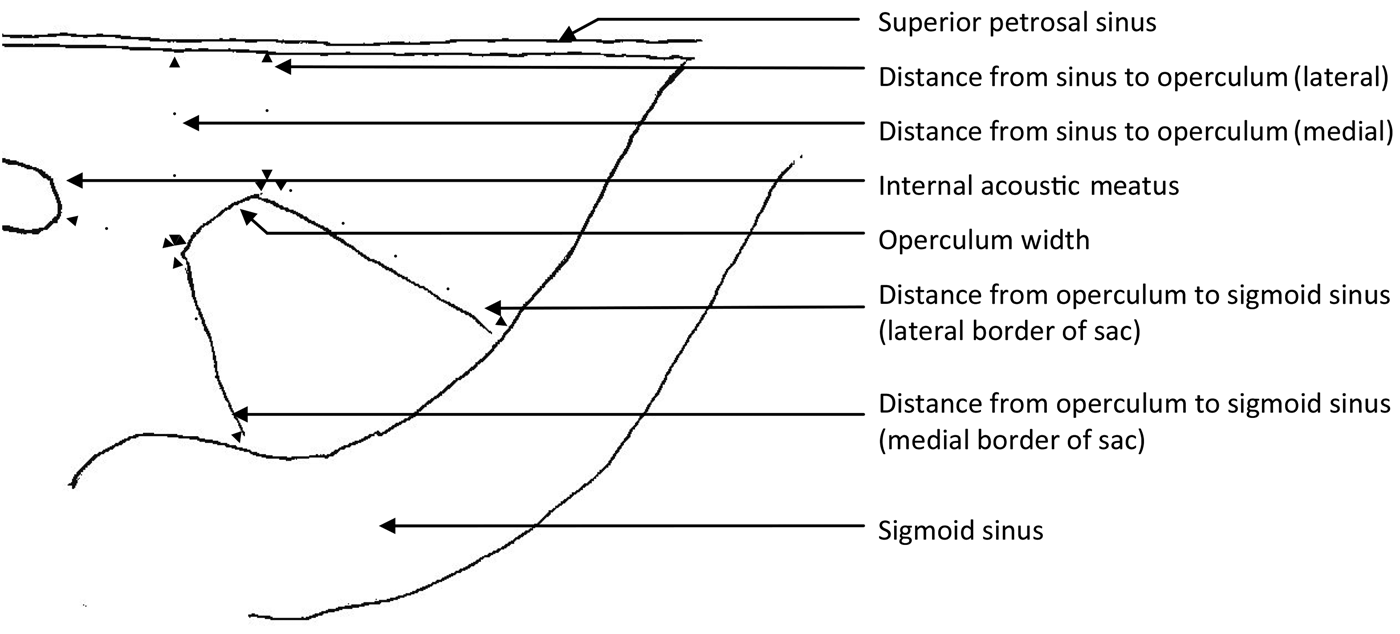
Fig. 1 Diagram of the posterior cranial fossa surface of a right petrous temporal bone, showing the measurements for endolymphatic sac length and width (measured at the widest point), and for the distances between: the superior petrosal sinus and operculum, the internal acoustic meatus and operculum, and the operculum and sigmoid sinus.
A careful note was kept of the ‘structures at risk’ during dissection.
Results
In the five simulated endolymphatic sac decompressions, there was a trapezoid thickening of dura in the posterior cranial fossa. This thickening lay in the endolymphatic fossa, and extended from a point medial to the posterior semicircular canal towards the sigmoid sinus laterally and jugular bulb inferiorly (Figure 2). The thickening was tethered at the operculum as the dura was elevated or ‘tented’ medial and lateral to this point. The thickening had a different colour when compared with the surrounding dura. Histology of the dural thickening revealed tubules of the sac. Deep to the sac, between the sac and the bone of the endolymphatic fossa, there were vascular structures which ran parallel to the sac (Figure 3).

Fig. 2 Dissection of right temporal bone performed via a transmastoid approach. A thickening in the dura corresponding to the endolymphatic sac can be seen. It extends from a point medial to the posterior semicircular canal towards the sigmoid sinus. Bone has been removed lateral to the facial nerve to improve exposure.

Fig. 3 Transverse section through the temporal bone at the level of the endolymphatic fossa, showing the thickening in the dura at this level and the underlying vascular structures. (H&E; ×10)
In the 14 topographical dissections of the posterior cranial fossa, the intradural part of the endolymphatic sac was identified lying in a similar trapezoid thickening of dura in the endolymphatic fossa adjacent to the operculum. The parallel edges of the trapezoid thickening were in the line of the posterior semicircular canal and the sigmoid sinus.
The operculum was relatively consistent in relation to the internal acoustic meatus. From the inferior limit of the operculum, the mean distance was 10 mm (range, 9–11 mm) (Table I). There was more variability between the operculum and the superior petrosal sinus. The mean distance from the inferior limit of the operculum to the sinus was 11 mm (range, 6–14 mm) and the mean distance from the superior limit was 10 mm (range, 6–13 mm).
Table I Endolymphatic sac measurements

Data represent measurements in mm. *Indicates that length of sac extended over the sigmoid sinus. †Not identified. M = medial; L = lateral; SD = standard deviation; CV = coefficient of variation
There was marked variability in terms of the size of the dural component of the endolymphatic sac. The mean width of the operculum was 5 mm (range, 2–7 mm) and the mean width of the sac at its maximal point was 12 mm (range, 7–16 mm). The mean length of the inferior border was 11 mm (range, 4–17) and the mean length of the superior border was 12 mm (range, 5–15 mm).
In eight cases, the endolymphatic sac extended beyond the medial margin of the sigmoid sinus, and in two cases no appreciable sac lumen was identified within the dura of the endolymphatic fossa (Table I). Scanning electron microscopy confirmed the flattened epithelial cells of the sac tubules and the surrounding fibrous tissue of the dura within the endolymphatic fossa (Figure 4).

Fig. 4 Scanning electron micrograph confirming the flattened epithelium of the intradural portion of the endolymphatic sac as distinct from the fibrous tissue of the dura on either side. (×500)
Discussion
The simulated operation revealed, in all specimens, a trapezoid dural thickening in the region of the endolymphatic fossa. The parallel lines of the trapezoid represent the posterior semicircular canal superiorly and the sigmoid sinus inferiorly, and the thickening extended to the jugular bulb inferiorly. The dural thickening appeared fixed at the operculum, with the dura adherent but able to be elevated medial and lateral to this point. The dural thickening also had a different colour from the surrounding dura. Histology confirmed that this thickening contained endolymphatic sac tubules and vascular structures in the fibrous tissue deep to the sac. The thickening was present even if the sac lumen was not identifiable.
Topographical dissection of the sac from the posterior cranial fossa revealed marked variability in the width and length of the intradural component. In some cases it extended beyond the medial margin of the sigmoid sinus, and in other cases no appreciable sac lumen was identified within the dura. There was great variability in the distance between the operculum (which is the origin of the intradural component of the sac) and the superior petrosal sinus. There was less variability in the distance between the operculum and the internal acoustic meatus. Scanning electron microscopy confirmed the presence of flattened epithelial cells lining the parts of the sac in the dura of the posterior cranial fossa. The structures at greatest risk when decompressing the sac were the posterior semicircular canal, the posterior cranial fossa dura and the sigmoid sinus.
All cadaveric material used in this research was embalmed by perfusion fixation. Although this provides good preservation of the material, it unfortunately also exposes the structures of the inner ear to abnormal pressures not unlike pathological hydrops. This may result in damage which is difficult to interpret; for example, expansion of the compartments of the inner ear and disruption of the separating membranes. As other studies have used material which was not perfusion-fixed, there may be artefactual differences.Reference Schuknecht and Gulya12
In temporal bone dissection, the endolymphatic sac is traditionally identified by three features: its white appearance, tenting of the sac or duct where it enters the vestibular aqueduct, and the presence of a lumen.Reference Sanna, Khrais, Falcioni, Russo and Taibah13, Reference Nadol and McKenna14 Our study was carried out on embalmed material, making direct correlation with colour and texture in life less reliable, but there is little doubt that the subtle differences in colour and flexibility of the embalmed dura correspond to the colour differences and tenting phenomenon seen at operation.
Our principal finding was a trapezoid thickening of the dura in the endolymphatic fossa, which was a reliable guide to the position of the endolymphatic sac. We believe this may be mistaken for the sac intra-operatively if there is no significant intradural component to the sac. The thickening appears to be tethered at the level of the operculum, and it may be that the fibrous tissue and vessels in this region, where the sac emerges from the vestibular aqueduct, fix the dura even if there is no intradural component to the sac.
Shea et al. used a combination of gross and histological methods to demonstrate the endolymphatic sac in 38 temporal bones.Reference Shea, Chole and Paparella15 They injected dye into the endolymphatic space to fill the sac, and then measured the sac and its relations. They located the dural component of the sac in every case; a mean width of 9 mm (range, 6–11 mm) and length of 9 mm (range, 6–12 mm) was reported. Sac sizes were similar to those of the present study. Bagger-SjöbäckReference Bagger-Sjöbäck16 and Friberg et al.Reference Friberg, Birgitta, Rask-Andersen and Bagger-Sjöbäck17 also describe variation in size using histological measurements. Friberg et al. did not locate the dural component in 2 out of 29 temporal bones. There was a larger variation in their raw data for total sac area than seen in other studies, with a coefficient of variation of 67.7 per cent. However, their mean reported sac width of 3.4 mm is considerably lower than that reported here (12 mm) or by Shea et al.,Reference Shea, Chole and Paparella15 which may reflect methodological differences. Friberg et al. also describe the sac extending beyond the medial margin of the sigmoid sinus in one-third of cases. However, in our study there were a higher number of cases in which the sac could not be identified.
In the topographical posterior cranial fossa dissections of the current study, the internal acoustic meatus and superior petrosal sinus were used as landmarks to find the operculum and the endolymphatic sac. However, from the transmastoid approach the internal acoustic meatus is not visible, but the superior petrosal sinus can be identified. Schuknecht and Gulya found the operculum approximately 10 mm from both the sigmoid sinus and the meatus.Reference Schuknecht and Gulya18 The findings of the present study are in agreement with these mean values, but it was clear that the meatus was a more reliable landmark with the lowest biological variation at 8.7 per cent. The sinus, which is identifiable at surgery, is more variable in distance from the sac, measuring approximately 10 mm (range, 6–13 mm) from the lateral limit of the operculum, with a biological variation of 23.4 per cent.
• Ménière's disease may be caused by endolymphatic hydrops; endolymphatic sac decompression has been advocated as a treatment
• A trapezoid thickening of the dura in the endolymphatic fossa marks the position of the sac
• Sac size was constant as it emerged medial to the operculum; intradural sac lumen varied in length and width, and was absent in 2 of 14 specimens
• Dissection may damage the sac, posterior semicircular canal, posterior cranial fossa dura and sigmoid sinus
• The trapezoid thickening of dura in the endolymphatic fossa was always present (even if sac was vestigial), and could be confused with the sac
• The sac should be decompressed as far proximally as possible whilst protecting the posterior semicircular canal
Shea et al. described using the tip of the short process of the incus to measure the lower limit of the posterior semicircular canal and thereby locate the endolymphatic sac.Reference Shea, Chole and Paparella15 From this, they suggest a more conservative decompression and staying lateral to the posterior canal. However, this is the area occupied by the intradural part of the sac. Our measurements show that this is the most variable portion; hence, a decompression confined to this area is not likely to be reliable in reaching the lumen. This may be particularly relevant in patients with Ménière's disease as it has been shown that the sac surface area is significantly smaller in such patients.Reference Bloch and Friis19
We recommend that bone is removed from the superior petrosal sinus to the jugular bulb, and from a point medial to the posterior semicircular canal to the sigmoid sinus. This exposes the proximal intradural portion of the sac and may decompress a segment of the intraosseous part. Given that the sac is more reliable in position proximally, decompression including the proximal region is likely to result in adequate decompression of the sac. It is likely that maximum decompression results in maximum benefit. It is clear, however, that decompressing the sac close to the posterior semicircular canal increases the risk of entering the latter and causing sensorineural hearing loss. These risks can be minimised if the anatomy is well understood and appropriate care is taken.
Conclusion
In the present study, simulated endolymphatic sac decompression confirmed a dural thickening in the region of the endolymphatic fossa in every case. Topographical dissection revealed that the intradural component of the sac was variable in size and position. In some cases, there was no identifiable lumen or the sac may have been so large as to overlie the sigmoid sinus. Hence, adequate decompression was not possible in every case. The trapezoid dural thickening was always present, even in the cases where the sac was vestigial and had no lumen within the dura. These variations may explain in part why the results of endolymphatic sac decompression operations are not consistent.
Surgery that avoids the posterior semicircular canal reduces the risk of sensorineural hearing loss but also reduces the chance of fully decompressing the endolymphatic sac. The most reliable way to decompress all the potential intradural component of the sac is to remove as much bone as safely possible from the endolymphatic fossa, from the posterior semicircular canal to the sigmoid sinus.