Introduction
Myringosclerosis may develop in the presence of acute or chronic middle-ear infection, and after myringotomy in the presence of serous middle-ear infection.Reference Tos and Stangerup1 Myringosclerosis is characterised by the hyalinisation and calcification of the collagen layer in certain areas of the tympanic membrane, and manifests itself as chalky white patches.Reference Tos, Bonding and Poulsen2 Although the aetiological mechanism for myringosclerosis is not clearly known, some studies have shown an effect of free radicals and mechanical damage.Reference Tos, Bonding and Poulsen2–Reference Ozcan, Polat, Gorur, Talas, Bagdatoglu and Cinel6 Thus, antioxidant or reductive agents may have the potential to reduce or prevent myringosclerosis development.
Laevo-carnitine is an antioxidant agent which prevents lipid oxidation or its final products.Reference Fabriello and Calabrase7 Carnitine is synthesised from the essential amino acids lysine and methionine. In humans, 75 per cent of carnitine is obtained from the diet. After its formation in the liver and kidneys, it is transferred to striated and cardiac muscle. Here, acetyl CoA acids are transferred into the mitochondrial matrix for β-oxidation of free fatty acids.Reference Flanagan, Simmons, Vehige, Willcox and Garrett8
This study aimed to investigate the effect of systemic and local administration of l-carnitine on the prevention of experimentally induced myringosclerosis, and to compare treatment efficiency, using histopathological analysis.
Materials and methods
Study design
The study protocol was approved by the Local Ethics Committee for Animal Experiments of Ankara University (approval number 21010-97-354).
The study used 24 healthy, adult, male, Albino-Wistar rats (48 ears) weighing 250–270 g, obtained from Hygiene Laboratory (Ankara, Turkey). The rats were kept at an appropriate temperature and humidity for 48 hours, and thereafter kept at 22 ± 2°C under 65–70 per cent humidity and ad libitum conditions in a 12-hour light–darkness cycle, at the Veterinary School of Ankara University. The study conformed to all the regulations of the Helsinki Declaration on experimental studies. Members of the study team monitored the rats without any knowledge of which group they belonged to, and performed evaluations based only on code numbers. Only the researcher responsible for the experiment was aware of the rats' code meanings and group identity.
All animals were bilaterally myringotomised (see below) and randomly divided into four groups prior to drug administration. Group one (control group; n = 6) received no treatment. Group two (n = 6) received 100 mg/kg intraperitoneal l-carnitine (as 1 g Carnitene; Santa Farma Pharmaceutical, Istanbul, Turkey). Group three (n = 6) received 20 mg/kg (0.1 ml) local l-carnitine. Group four (n = 6) received both intraperitoneal and local l-carnitine.
On the 15th day after treatment, the rats were sacrificed and their tympanic membranes removed. The tympanic membranes were histopathologically examined to evaluate myringosclerotic plaque formation, fibroblastic proliferation, tympanic membrane thickness and new vessel formation.
Details of the experimental intervention were as follows. Animals were anaesthetised with 60 mg/kg intraperitoneal ketamine hydrochloride (Ketalar, Eczacibaşi Parke-Davis, Istanbul, Turkey) and 10 mg/kg intraperitoneal xylazine HCl (Alfazyne, Alfasan International, Woerden, Holland). Each rat's external and middle ears were examined with an otomicroscope. Ears with cerumen were cleaned. Any rats with opacity, perforation or infection in the external and/or middle ear were excluded from the study. The rats' rectal temperatures were regularly monitored, and they were placed on a heated blanket to keep their body temperatures at 35°C. The tympanic membrane was perforated under sterile conditions, using a myringotomy knife, under otomicroscopic guidance. A 1–1.5 mm piece of sponge (GelSpon-P, Eucare Pharmaceuticals, Chennai, India) was inserted into the external ear canal. Local l-carnitine was then applied. The various experimental treatments were continued for 15 days. At the end of the treatment period, rats were sacrificed under the supervision of the main researcher (who was certified in handling experimental animals) and a veterinarian.
Outcome measures
Specimens were evaluated by two pathologists who were blinded to group allocation, and a consensus reached.
Specimens were prepared as follows. The rats' tympanic membranes were removed under otomicroscopic guidance, taking care to preserve the unity of the membranes (Figure 1). The tympanic membranes were fixed in 10 per cent formaldehyde and then kept in 10 per cent formic acid solution for 24 hours; in this way, all were decalcified. After routine paraffin processing, 5-µm sections were taken and stained with haematoxylin and eosin.

Fig. 1 Photograph of tympanic membrane tissue excised from the middle ear.
The sections were then evaluated semi-quantitatively, using a Leica DMLB light microscope (Leica Microsystems, Wetzlar, Germany) (×20 objective magnification, ×0.40 ocular magnification), noting the following parameters: sclerosis severity, fibroblastic proliferation rate and new vessel formation. The thickness of the tympanic membrane was determined using an ocular piece which superimposed a 100 unit space grid, with each unit equivalent to 5 µm2, under ×20 magnification. In cases with heterogeneous thickening of the tympanic membrane, the thickest area was measured. Myringosclerosis was categorised as none (scored as 0), scarce (i.e. a few lesions on the lamina propria; scored as 1), moderate (single or combined lesions on the lamina propria; scored as 2) or severe (large, combined lesions on the lamina propria; scored as 3), based on semi-quantitative definitions. Fibroblast proliferation density was defined as none (scored as 0), mild (+; 1), moderate (++; 2) or severe (+++; 3). New vessel formation was defined as none (scored as 0), mild (+; 1), moderate (++; 2) or severe (+++; 3).
Statistical analyses
Data were analysed using the Statistical Package for the Social Sciences (SPSS) software program (version 11.0 for Windows). All differences associated with a chance probability of 0.05 or less were considered statistically significant. The distribution of continuous variables was analysed using the Shapiro–Wilk test. Descriptive statistics for tympanic membrane thickness were shown as median values and inter-quartile widths, while those for myringosclerosis, fibroblast proliferation density and new vessel formation were shown as medians and ranges. The significance of differences between the median values of the groups was analysed using the Kruskal–Wallis test. Statistically significant differences were further analysed using the non-parametric multiple comparison test, to determine the factor(s) responsible for the difference. The presence or absence of significant correlations between tympanic membrane thickness, myringosclerosis and fibroblast proliferation density was determined using Spearman's correlation test.
Results
One rat each from group one (control group) and group two (intraperitoneal l-carnitine) died, on days 4 and 7, respectively, and were therefore excluded from the study.
Tympanic membrane thickness results were as follows: 137.50 ± 61.11 µm in group one, 127.50 ± 61.11 µm in group two, 143.33 ± 59.70 µm in group three and 102.08 ± 37.68 µm in group four. There was a statistically significant increase in the tympanic membrane thickness in group one compared with the other groups (p < 0.05). The difference in tympanic membrane thickness between groups three and four was also statistically significant (p < 0.05).
Regarding myringosclerosis severity and fibroblast proliferation density, no statistically significant difference was found between groups one and three (p > 0.05); however, these parameters were significantly more severe in groups one and three compared with groups two and four (p < 0.05; Figures 2 and 3).
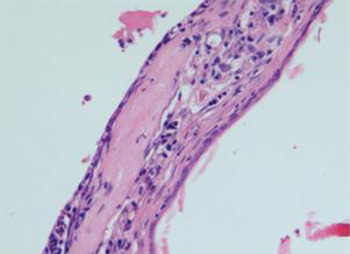
Fig. 2 Photomicrograph of tympanic membrane showing moderate myringosclerosis. (H&E; ×20)
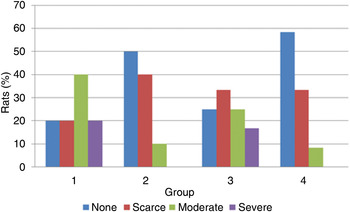
Fig. 3 Severity of myringosclerosis in each experimental group.
No statistically significant difference was found between groups two and four for any of the parameters studied (p > 0.05; Table I).
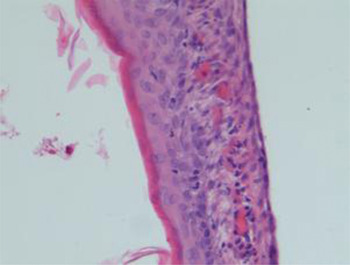
Fig. 4 Photomicrograph of tympanic membrane showing prominent neovascularisation. (H&E; ×20)
Table I Histological results

Statistically significant differences were seen between: *groups one and two (p < 0.01); †groups one and three (p = 0.040); ‡groups one and four (p < 0.01); §groups three and four (p < 0.05); and #groups two and three (p < 0.05). See text for explanation of scoring systems. Med = median; IQW = inter-quartile width
All groups had similar results for new vessel formation (p > 0.05; Figure 4). When we evaluated associations between tympanic membrane thickness, myringosclerosis severity, fibroblast proliferation density and new vessel formation, we found significant correlations between tympanic membrane thickness and myringosclerosis severity and fibroblast proliferation density (p < 0.05; Table II), but not between tympanic membrane thickness and new vessel formation (p > 0.05).
Table II Correlation between tympanic membrane thickness and other variables

Discussion
In the presented animal model, intraperitoneal l-carnitine administration was found to be effective in preventing myringosclerotic lesions and reducing tympanic membrane thickening.
Myringosclerosis develops as a sequel of acute or chronic, recurrent middle-ear infection, and can also develop after myringotomy in the presence of serous middle-ear infection. Although the morphology of myringosclerosis has been well described, its exact pathogenesis is not clearly established. Several aetiological hypotheses have been proposed, including reactive oxygen species, immunological sensitivity, mechanical injury and metabolic disturbance. After myringotomy, an inflammatory reaction develops in the normal middle ear and tympanic membrane. Polymorphonuclear cells and macrophages then migrate into the inflammation site, leading to phagocytosis and elevated arachidonic acid metabolism. Increased quantities of superoxide radicals are produced, which may contribute to the development of myringosclerosis.
In a study by Mattson et al., myringotomised rats were exposed to different concentrations of oxygen.Reference Mattsson, Magnuson and Hellström9 Greater numbers of myringosclerotic lesions were found in the tympanic membranes of myringotomised rats exposed to higher oxygen concentrations. These authors also showed that a combination of copper zinc superoxide dismutase plus catalase and deferrioxamine inhibited or reduced the development of myringosclerosis.
Various agents have previously been used to prevent myringosclerosis development. Spratley et al. found a reduced occurrence of myringosclerosis following topical ascorbic acid application.Reference Spratley, Hellstrom, Mattsson and Pais-Clemente4 Ozcan and colleagues reported a lower rate of myringosclerosis development in a study group treated with topical N-acetylcysteine, compared with controls.Reference Ozcan, Gorur, Cinel, Talas, Unal and Cinel5, Reference Ozcan, Polat, Gorur, Talas, Bagdatoglu and Cinel6 Song et al. used caffeic acid phenethyl ester, and found less myringosclerosis and thicker tympanic membranes in their treatment group compared with controls.Reference Song, Kwon, Cho and Park10 Akbaş et al. used l-carnitine and obtained similar results.Reference Akbaş, Pata, Gorur, Polat, Polat and Ozcan11 Emir et al. used Ginkgo biloba extract, and reported a reduction in tympanic membrane thickness and myringosclerosis development rates.Reference Emir, Kaptan, Samim, Sungu, Ceylan and Ustun12 Gorur et al. used systemic selenium and reported decreased myringosclerosis formation in rat ears.Reference Gorur, Ozcan, Polat, Unal, Tamer and Cinel13
In our study, we observed a lower rate of myringosclerosis development following local plus intraperitoneal administration of l-carnitine, and also following intraperitoneal use alone. Tympanic membrane thickness was lower in the study group than the control group. In the group treated with local l-carnitine, the myringosclerosis development rate did not differ significantly between the study and control groups. Combined intraperitoneal and local l-carnitine administration was not observed to have any additional benefits as regards myringosclerosis prevention.
• This study assessed whether local and/or intraperitoneal l-carnitine affected myringosclerosis in a rat myringotomy model
• Local l-carnitine did not prevent myringosclerosis or tympanic membrane thickening
Following acute tympanic membrane perforation, tympanic membrane thickness increases due to oedema in the fibrous layer, inflammation and new vessel formation. The tympanic membrane also shows increased fibroblastic activity and smooth-celled epithelium proliferation.Reference Güneri, Tekin, Yilmaz, Ozkara, Erdağ and Ikiz14, Reference Johnson, Smallman and Kent15 Ovesen et al. evaluated fibroblast activation and proliferation in the middle ears of rabbits, and found that the use of N-acetylcysteine reduced the rate of fibroblast proliferation and activation, which in turn led to a decrease in tissue thickness.Reference Ovesen, Gaihede and Ledet16 Ozcan et al., however, did not observe a decrease in the rate of fibroblast proliferation or activation.Reference Ozcan, Gorur, Cinel, Talas, Unal and Cinel5 In our study, reduced fibroblast proliferation was seen following daily application of l-carnitine, both for intraperitoneal and for combined intraperitoneal and local administration. A correlation was found between tympanic membrane thickness and both fibroblastic proliferation rate and myringosclerosis severity. No significant difference was found between the groups as regards new vessel formation, and there was no significant correlation between tympanic membrane thickness and new vessel formation.
Carnitine reduces the effect of free radicals via various routes, and plays an important role in the transportation of free fatty acids from the cytoplasm into mitochondria. Free fatty acids are reduced to acetyl CoA by β-oxidation and enter into the tricarboxylic acid cycle. A large amount of oxygen is consumed during this reaction, driving the electron transport chain and resulting in adenosine triphosphate synthesis and oxidative phosphorylation. The resulting reduction of oxygen to H2O prevents free radical formation.Reference Akbaş, Pata, Gorur, Polat, Polat and Ozcan11, Reference Mayes, Murray, Granner, Mayes and Rodwell17
Conclusion
The rate of myringosclerosis development is significantly reduced in myringotomised rats treated with intraperitoneal l-carnitine. However, the effectiveness of local l-carnitine in this setting appears to be limited. Intraperitoneal l-carnitine also ameliorates myringotomy-associated morbidity by reducing fibroblastic proliferation and tympanic membrane thickening, both of which cause further destruction of tympanic membrane structure.
Although these results suggest that the use of l-carnitine may reduce myringosclerosis development following myringotomy and/or ventilation tube insertion in humans, effects may vary in clinical practice. Therefore, further studies, particularly clinical trials, should be conducted to investigate the potential benefits of l-carnitine application in humans.








