Introduction
The French physician Pierre Bretonneau gave the name diphtheria to the disease that had been well known since ancient times. Originally a Greek word, the term refers to the leathery pseudomembrane which grows on the pharynx, tonsils and nose, resulting in mechanical obstruction of the airway. Throughout history, epidemics of diphtheria have wiped out hundreds of thousands of children,Reference Amoros-Sebastia, Ferrer-Baixaulim, Salavert-Fabiani and Lopez-Martinez1 regardless of socio-economic class. Queen Victoria lost her daughter and granddaughter to the disease. In 1890, the first Nobel prize in medicine was awarded to the German physician Emil von Behring for the discovery of diphtheria antitoxin.Reference Lindsten2 The first vaccines were developed in the late 1930s. In 1942, mass vaccination commenced in the UK. Since that date, there has been an almost complete cessation of new cases of diphtheria, to such an extent that most clinicians currently practising will not have seen a single case throughout their careers. However, following the collapse of the Soviet Union, mass vaccination programmes were partially abandoned in the former countries of the USSR, with disastrous results. By the end of the 1990s, thousands had died, and the disease soon spread to other European countries, resulting in further fatalities.Reference Wren and Shetty3
Despite this renewed threat, classic diphtheria has remained a rare disease in the UK. While newer vaccination campaigns in the UK are aimed at increasing the level of protection against diphtheria, contact with developing parts of the world, via rapid air travel, will continue to remind clinicians of this deadly disease.
We report a case of diphtheria treated in our department, and its fatal outcome.
Case report
A 76-year-old hypertensive female patient presented to the accident and emergency department of our hospital with a five-day history of non-productive cough, mild pyrexia and profuse, purulent nasal discharge that had rapidly became sanguinous. In the 24 hours preceding admission, she had noticed a rapid deterioration in her ability to talk due to progressive swelling of her soft palate, and decreased nasal airflow.
On admission, the patient had a temperature of 37.8°C and a grossly swollen palate. She was treated promptly with intravenous hydrocortisone, chlorpheniramine and benzyl penicillin. However, as she had recently been commenced on an angiotensin-converting enzyme inhibitor, it was felt that her palatal oedema was possibly angioneurotic in origin, and the relevant medication was stopped.
Overnight, the patient seemed to improve; however, by midday, it became apparent that the oedema of her nose and palate had progressed. No focus of infection or pseudomembrane was visible at this stage. The patient's nasal mucosa had swollen to such an extent that even a senior ENT surgeon could not pass a flexible nasendoscope.
Computed tomography of the patient's paranasal sinuses and neck confirmed soft tissue swelling of her nose, nasopharynx and palate, without obstruction of the trachea or larynx (Figure 1).

Fig. 1 Axial computed tomography scan of the oral cavity, showing gross oedema of the soft palate and uvula.
Despite treatment with intravenous Augmentin® and oral prednisolone, the patient's airway continued to deteriorate, and a tracheostomy became necessary that evening. During endoscopy, a thin, friable, yellow-grey membrane lining the pharynx and nose became evident for the first time (Figure 2).
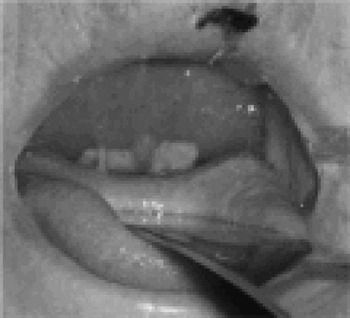
Fig. 2 Picture of the pseudomembrane lining the palate, showing thick palatal oedema.
While none of the clinicians involved had seen a case of diphtheria, the possibility of the diagnosis was considered. Throat and nasal swabs and blood cultures were immediately sent for culture and sensitivity analysis, and the on-call microbiologist was notified. The same night, both the Communicable Disease Surveillance Centre and the microbiology department were notified and antitoxin was requested. While awaiting confirmation of the diagnosis, the patient was treated as a case of diphtheria, with diphtheria antitoxin in addition to intravenous benzyl penicillin, erythromycin and metronidazole.
Public health officials were notified. Family members and medical personnel who had been in close contact with the patient were screened for diphtheria and treated with erythromycin orally for one week. Booster vaccinations were administered where appropriate. While initial throat swabs did not yield diphtheria species, the diagnosis of diphtheria ulcerans was made based on polymerase chain reaction analysis of the slough obtained from the patient's pharynx at original endoscopy.
The patient was nursed in the intensive care unit, with respiratory support and barrier nursing. After tracheostomy, her clinical course became more turbulent. Generally, she remained toxic with a hyperdynamic circulation, and required ventilatory support. While her initial renal function had been poorer than normal, this deteriorated daily.
The patient developed multiple vesicles filled with straw-coloured fluid on her chest wall, associated with a large area of intervening cellulitis. Increasing amounts of rubbery, necrotic material were removed from her palate. This material was adherent to the mucous membranes of her nose and oropharynx. Attempts at removing it resulted in mucosal bleeding. Nevertheless, removal of the necrotic material from the oropharynx and the tracheostomy tube contributed to the patient's comfort.
After a few days, the patient developed acute renal failure, followed within 24 hours by a massive myocardial infarction confirmed by raised troponin levels and echocardiography. In view of the large size of the infarction, it was decided not to treat the patient aggressively. The patient died very soon after the myocardial infarction, approximately two weeks after the initial onset of her symptoms.
Discussion
Corynebacterium diphtheriae, and the lesser-involved C ulcerans, are gram-positive, aerobic bacilli which are non-encapsulated and non-spore-forming. Corynebacterium diphtheriae gains the ability to produce its toxin once it has been infected by a virus carrying the tox gene. Spread by droplets after an incubation period of 2–5 days, the classic respiratory version of diphtheria leads to pyrexia, sore throat, a possible pseudomembrane on the tonsils, pharynx and even in the nose, and cervical lymphadenopathy.
The local effects of the toxin include tissue destruction (typically, lower limb vesicles which ulcerate). Systemically, the toxin leads to life-threatening complications such as myocarditis, demyelinating polyneuropathy, thrombocytopenia, proteinuria and renal failure. The disease can also cause death due to airway obstruction by the pseudomembrane in the oropharynx. The exotoxin consists of two polypeptide chains linked by disulphide bonds. There are four types of C diphtheriae – gravis, intermedius, mitis and belfanti, with the gravis and mitis types being most commonly involved in clinical infection.Reference MacGreggor, Mandell, Bennett and Dolin4–Reference Efstratiou and George6
Non-toxogenic strains lead to much milder forms of infection. These strains have attracted more attention in recent years as they can lead to recurrent pharyngitis. While the exact mechanism of their action remains unknown, as does their epidemiological significance, they have rarely been shown to cause a tonsillar membrane or endocarditis.Reference Peters7–Reference Tiley, Kocioba, Heron and Munro9
In Western countries, carriers of the bacterium have become extremely rare following the advent of vaccination. These carriers can be treated with oral penicillin, erythromycin, clarithromycin and azythromycin.Reference Bonnet and Begg10 After the onset of mass immunisation programmes in the early 1940s, it had been hoped to totally eradicate diphtheria as a human disease. However, in lesser-developed parts of the world, diphtheria continues to be a real threat to the survival of children. Furthermore, the breakdown of vaccination services at the end of the Soviet era prompted a significant resurgence in diphtheria cases.Reference Ditmann, Wharton, Vitek, Ciotti, Galazica and Guichard11
In the UK, vaccination programmes started in 1942. Since that time, classic diphtheria has almost disappeared, resulting in very little exposure of medical staff to the disease. However, a high level of suspicion is still required to enable prompt diagnosis of the disease.
Over the past few years, changes in the vaccination protocol in England and Wales have been designed to further reduce the possibility of diphtheria outbreaks. These steps have included an accelerated schedule of diphtheria–pertussis–tetanus vaccination in the 1990s, the addition of diphtheria to the school-leaving tetanus dose, and, most recently, the combination of cellular components of the diphtheria–pertussis–tetanus vaccine to the meningoccocal C vaccine used to vaccinate preschool children (as well as individuals travelling to less developed regions of the world).Reference Maple, Jones, Wall, Vyseb, Edmunds and Andrews12–Reference Anonymous15
In general, the level of immunity in vaccinated populations falls with age, and this effect is more pronounced in women. Reasons may include lack of vaccination for the older generation, and a fall of immunity with advanced years. This has led to reinforced calls for booster doses of the vaccine every 10 years for adults over 21 years. Only 30 per cent of those aged over 60 years have adequate immunity against diphtheria. The discrepancy between male and female immunity may be explained by the use of diphtheria toxoid in army recruits. This age and sex difference in immunity levels has also been observed in other developed countries such as the USA and Sweden.Reference Christenson and Bottinger16–Reference Ruben, Nagel and Fireman22
The number of reported cases of diphtheria in the UK remains low. Between 1990 and 1996, only 18 cases were reported. Most of these individuals had returned from travel abroad, and a smaller group had had contact with travellers returning from countries where diphtheria is endemic.Reference Anonymous23 Furthermore, between 1995 and 2002, 17 patients with cutaneous diphtheria were reported: all had returned from travel abroad. As the numbers affected by toxogenic strains of C diphtheriae remain low in the UK, diagnosis can be difficult, resulting in delay in initiating treatment.
• Diphtheria still exists in the UK, and can lead to rapid swelling and obstruction of the airway in any age group
• Upon suspicion of acute diphtheria, the ENT surgeon must instigate immediate measures to secure the airway and treat the infection
• Microbiology department consultants and the Centre for Communicable Disease Control must be notified at the earliest possible opportunity
• Prompt patient isolation, contact tracing, testing and treatment are crucial to management
The causative organism isolated from our patient (C ulcerans) has the ability to infect the udders of cows. Transmission to humans by drinking raw milk has been documented; however, no direct evidence of person-to-person transmission has been found.Reference Meers24–Reference Bostock, Gilbert, Lewis and Smith26
According to the Infectious Diseases (Notification) Act of 1889, diphtheria is a notifiable disease. Upon suspicion of a case of diphtheria, the responsible clinician should inform a communicable disease control consultant, seek advice from a health protection agency microbiologist, and discuss the public health impact of the case with the Communicable Disease Surveillance Centre. Prompt isolation of the index case and tracing of close family contacts and medical staff involved in intimate examination of the patient (in order to take nasal and throat swabs) should be followed by administration of prophylactic antibiotics, such as erythromycin, and possible booster vaccination. Recently, the introduction of polymerase chain reaction analysis has greatly reduced the waiting time necessary to reach a diagnosis.Reference Mothershed, Cassidy, Pierson, Mayer and Popvic27, Reference Efstratiou and George28
Our 76-year-old patient and her family had no known history of diphtheria vaccination. She lived alone in a town but had recently visited a farm. She had not consumed unpasteurised milk or milk products. There was no known history of travel to affected areas. While a close family contact had originated in Eastern Europe, the family screening tests were negative. There were no other, subsequent reports of diphtheria in the patient's community. Interestingly, one of the family dogs screened positive for C diphtheriae. However, as the strain was not C ulcerans, this seems unlikely to have been the source of infection.
Conclusion
Infections with C diphtheriae and C ulcerans are rare in the UK. Their diagnosis depends upon a high degree of clinical suspicion. With the advent of rapid air travel, more cases may come to the attention of the otolaryngologist. Prompt treatment of the index case and screening of close contacts remain the mainstays of management.
Acknowledgement
The authors would like to thank Dr J Hart for her kind suggestions in the writing of this case report.




